Feeding DDGS diets impact swine health
Part 14 in the series “What have we learned about feeding distillers co-products to pigs over the past 20 years?”
October 30, 2019

By Jerry Shurson, University of Minnesota Department of Animal Science
Regulations that have led to restricting antibiotic use for growth promotion have caused swine nutritionists consider various feed additives, functional ingredients and functional nutrients that can be alternatively used to promote growth and health of pigs. Compared with other grain byproducts, dried distillers grain with solubles has relatively high concentrations of natural antioxidant compounds that may contribute toward reducing oxidative stress.
Furthermore, DDGS contains about 10% spent yeast, of which yeast cell walls contain β-glucans, mannanoligosaccharides and nucleotides that have been shown to provide beneficial effects on swine health and growth performance. The relatively high concentration of insoluble fiber in DDGS may affect gut health through changes in cell proliferation, digesta viscosity and microbiome, but limited studies have been conducted to characterize these effects. Although the direct effects of antioxidants, yeast cell wall components, and fiber on swine health is not well understood, there are a few studies that have shown gut health benefits for specific pathogens when feeding DDGS diets to pigs.
Finally, a new dimension of sourcing feed ingredients involves assessing the relative risk of transmission of foreign animal diseases, such as porcine epidemic diarrhea virus and African swine fever virus. Preliminary data suggest that compared to soybean meal and corn, DDGS is a lower risk factor for PEDV survival, and does not appear to be a significant risk factor for ASFV transmission.
Installments in the DDGS series
Part 1: 20 years of DDGS lessons in pig diets
Part 2: Varied energy and digestible amino acids levels in DDGS manageable
Part 3: Work continues to evaluate performance responses from feeding DDGS
Part 4: Managing carcass yield, pork fat quality when feeding corn DDGS
Part 5: Reaching an understanding of fiber characteristics of corn DDGS
Part 6: Enzymes, pre-treatment improve fiber and nutrient digestibility
Part 7: DDGS show greater antioxidant capacity than in corn grain
Part 8: Need better understanding of energy levels in distillers corn oil
Part 9: Corn DDGS a good source of digestible phosphorus for swine
Part 10: Feeder design and diet management impact performance with DDGS diets
Part: 11: Feeding DDGS diets to gestating and lactating sows
Part 12: DDGS present handling and storage considerations
Part 13: Pelleting DDGS diets has benefits, drawbacks
Part 14: Feeding DDGS diets impact swine health
Phytochemicals and antioxidant properties of DDGS
Several studies have been conducted to determine the concentrations of phytochemicals and antioxidant capacity of DDGS. Winkler et al. (2007) used several different extraction methods to quantify the phytosterols (1.97 to 2.91 mg/g of DDGS), phytostanols, total tocopherols and tocotrienols (0.73 to 1.82 mg/g of extract), and ferulate phytosterol esters (0.35 to 0.53 mg/g of DDGS) in corn oil and corn fiber oil from DDGS. In a subsequent study, Winkler-Moser and Breyer (2011) determined the oxidative stability, tocopherols, tocotrienols, carotenoids, phytosterols and steryl ferulates in corn germ oil and oil extracted from post-fermentation fractions and DDGS. They found that hexane extracted oil from DDGS was the most oxidatively stable and had significant quantities of functional lipids that serve as antioxidants to increase the oxidative stability.
Luthria et al. (2012) also determined the phenolic (vanillic, caffeic, p-coumaric, ferulic and sinapic) acid concentration and antioxidant capacity of DDGS and compared those concentrations to those in corn grain. They found that the phenolic acid profile in DDGS was similar to corn, with ferulic and p-coumeric acids representing about 80% of the total phenolic acids. Furthermore, the total phenolic acid content in DDGS was 3.4 fold greater, and antioxidant capacity was 2.6 fold greater than that in corn grain. In a subsequent study, Luthria et al. (2014) reported that the increased phenolic acid concentration in DDGS was mainly due to depletion of starch during the ethanol fermentation process.
More recently, Shin et al. (2018) evaluated the antioxidant capacity, tocopherols, tocotrienols, xanthophylls and ferulic acid content of 16 sources of DDGS and compared those values with corn grain (Table 1). Antioxidant capacity of DDGS sources ranged from 29 to 65 mmol tocopherol equivalent per kg. The total tocopherols ranged from 91 to 141 mg/kg, total xanthophylls ranged from 447 to 1,586 mg/kg, and total ferulic acid content ranged from 6.8 to 9.5 mg/kg. As expected, DDGS sources had much greater concentrations of these important functional lipids than corn grain. Tocopherols, carotenoids (xanthophylls) and ferulic acid are strong antioxidant compounds that appear to be beneficial in preventing greater oxidation during thermal exposure, which occurs during the DDGS production and drying processes. Furthermore, the relatively high concentrations of these compounds in corn oil from DDGS appear to beneficial in minimizing oxidative stress when feeding highly oxidized DDGS sources to nursery pigs (Song et al., 2013; Hanson et al., 2015b) and wean-finish pigs (Song et al., 2014).
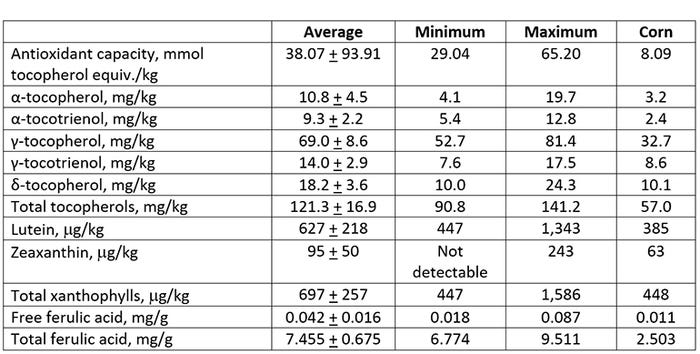
It is important to determine the extent of lipid oxidation and antioxidant capacity in feed ingredients, especially those with relatively high concentrations of lipid (e.g. DDGS), because it can affect the potential for further oxidation, storage stability and the need for adding supplemental antioxidants. Hanson et al. (2015a) showed that adding either ethyoxyquin or TBHQ antioxidants were about 50% effective in preventing further oxidation from occurring when DDGS samples were stored under hot, humid conditions. Recent reviews have been published regarding the complexity of measuring and interpreting lipid oxidation data (Kerr et al., 2015; Shurson et al., 2015). However, a meta-analysis of several studies has shown significant reductions growth performance and metabolic oxidation status in pigs fed oxidized lipids (Hung et al., 2017).
Although results from previous studies (Winkler et al., 2007; Winkler-Moser and Breyer, 2011; Luthria et al., 2012; Shin et al., 2018) consistently showed that DDGS has high antioxidant capacity, Song and Shurson (2013) evaluated the extent of corn oil oxidation in 31 corn DDGS sources. Their results showed that oil oxidation occurs during the DDGS production and drying process, and the corn oil in some sources may be up to 25 times more oxidized than found in corn grain. In a subsequent study, Song et al. (2013) fed zero or 30% DDGS diets containing three dietary levels of supplemental vitamin E (none, at the National Research Council requirement, and 10 times the requirement) to evaluate the oxidative status of weaned pigs. The DDGS source used in this study was the most oxidized source identified in the Song and Shurson (2013) study, which also contained 0.95% sulfur. Results from this study showed that feeding the DDGS diet increase serum α-tocopherol and sulfur-containing amino acid concentrations, liver glutathione concentration and glutathione peroxidase activity. They concluded that the increased concentrations of sulfur-containing antioxidants (methionine, taurine and glutathione) may protect pigs against oxidative stress when fed highly oxidized DDGS, and that increasing dietary vitamin E concentrations may not be necessary.
Several years ago, a few veterinarians were observing an increased incidence of Mulberry heart disease (vitamin E and selenium deficiency) in nursery pigs and attributed these observations to feeding DDGS diets. Therefore, Hanson et al. (2015b) conducted a study to determine if feeding diets containing the most oxidized DDGS source (identified by Song and Shurson, 2013), with or without supplemental vitamin E (five times the requirement), to gestating and lactating sows and their offspring through the nursery period, caused an increase in MHD during the nursery period. Pigs fed the 30% oxidized DDGS diet had increased feed intake, but average daily gain was not affected compared to those fed the corn-soybean meal control diet.
Furthermore, feeding the oxidized DDGS diet, with or without high vitamin E supplementation, increased serum α-tocopherol concentrations, but there were no differences in glutathione peroxidase activity and TBARS concentrations, nor were there any histopathological lesions indicative of MHD when pig hearts were examined. Similar to the results reported by Song et al. (2013), the serum concentrations of total sulfur-containing amino acids (cystathionine, cysteine, methionine and taurine) were increased by 40 to 50% in pigs fed the oxidized DDGS diets. Sulfur-containing amino acids have metabolic antioxidant properties and can counteract oxidative stress by acting as reducing agents. Results of this study suggest that the increased intake of sulfur-containing amino acids, vitamin E and other antioxidant compounds in DDGS prevented the development of MHD and the potential negative effects of feeding oxidized DDGS.
Weber and Kerr (2011) evaluated the effects of feeding DDGS diets on oxidative stress and immune function of growing pigs. They found no effects on circulating metabolic oxidative stress indicators from feeding a 35% DDGS diet, but showed an increase in plasma IgA and IgG, suggesting an improvement in humoral immunity in finishing pigs.
In contrast, Li et al. (2012) reported that feeding DDGS diets to growing-finishing pigs reduced plasma and tissue redox status. However, Song et al. (2014) showed no benefit of adding supplemental vitamin E to highly oxidized DDGS diets fed to wean-finishing pigs on growth performance. Therefore, the majority of studies indicate that feeding diets containing DDGS with varying amounts of oxidized lipids has no detrimental effect on growth performance, and may enhance humoral immunity and minimize oxidative stress due to increased antioxidant compounds in DDGS.
Yeast content in DDGS
Significant amounts of yeast are added to fermenters during the ethanol production process, and spent yeast remains in co-product streams used to produce DDGS. Yeast cell walls contain β-glucans, mannanoligosaccharides and nucleotides, which are well known nutraceutical compounds that have generally been shown to improve animal growth performance and health (Shurson, 2018). However, it is difficult to quantify the concentration of spent yeast, and yeast cell wall components in DDGS (Shurson, 2018). A few researchers have attempted to estimate the yeast content in DDGS, but the methods used have several limitations and the results reported have been highly variable.
Ingledew (1999) used a mass balance approach to estimate that 3.9% of the total DDGS biomass, and 5.3% of the total protein content was derived from yeast. Belyea et al. (2004) estimated the yeast content of DDGS by calculating the proportional contributions of amino acids from yeast and corn relative to the total amino acid content in DDGS. These researchers estimated that about 50% of the protein in DDGS was derived from yeast. However, this method ignored the effect of amino acid composition in corn, which resulted in an inaccurate estimate of yeast content in DDGS. Han and Liu (2010) used a more detailed approach by conducting a multiple linear regression analysis using the relative percentage of individual amino acids in corn and yeast, relative to total amino acid content of DDGS. They suggested that about 20% of DDGS protein is derived from yeast. However, they acknowledged that this estimate may not be accurate because it assumes that 20% of DDGS biomass is comprised of yeast, which is not accurate. Alternatively, it is possible to use the mannan content in yeast cell walls to calculate an estimate of yeast content in DDGS because mannans are only found in yeast cell walls and not in corn grain (Shurson, 2018). Using this method, the estimated yeast content of DDGS is about 10%, and the yeast content of high-protein corn co-products may be as high as 29% yeast, depending on the production method used (Shurson, 2018).
Yeast cell walls comprise about 15 to 20% of the dry weight of yeast cells, and the main polysaccharide fractions are β-glucans and mannans. The β-glucans have been shown to adsorb or bind toxins, viruses and pathogenic bacteria (Vetvicka et al., 2014). Immune cells (macrophages) have receptors for β 1,3/1,6 branched glucans, and their mode of action has been well defined in human nutrition and medicine (Rop et al., 2009). There is also research evidence showing that dietary β-glucans may improve immune-competence in young animals (Saeed et al., 2014). Lim et al. (2009) used an enzymatic method designed to measure β-glucan content in barley and oats, and estimated that the β-glucan content of DDGS is about 0.57%. However, it is uncertain if this method is suitable for use in estimating the β-glucan concentration in DDGS. Kim et al. (2008) determined the total glucan concentration in DDGS to be about 21.2% (dry matter basis), of which 16% was attributed to cellulose and 5.2% was from starch. Glucans are present in bacteria, fungi, yeast and cereal grains, but vary in molecular configuration, solubility and functionality. Furthermore, cellulose is not considered a functional source of β-glucans because it is insoluble and does not provide the same functionality of yeast β-glucans (Sikora et al., 2013). No other studies have been conducted to estimate the β-glucan content in DDGS, but recent estimates using a Megazyme International Yeast β-glucan assay showed that the β-glucan content in a high-protein DDG was about 8.3% (Shurson, 2018).
Mannanoligosaccharides (MOS) function as prebiotics (sources of nutrients for certain microbes in the gastrointestinal tract), that can subsequently result in providing a probiotic effect (Spring et al., 2015). Spring et al. (2015) reviewed results from numerous published studies that evaluated feeding MOS supplemented diets to multiple animal species, and results generally showed improvements in growth rate, feed conversion and reductions in mortality. These researchers suggested that these improvements are a result of MOS binding and limiting the colonization of pathogens in the gastrointestinal tract, improving the integrity of the intestinal mucosa, modulating immune system activity, and may be involved in antioxidant and anti-mutagenic defenses. Alizadeh et al. (2016) used a modified procedure to determine the mannose content of a wheat-corn DDGS source and reported it contained 1.6% mannose.
Yeast contains significant amounts of nucleotides, with concentrations of total nucleic acids reported to be between 3.3 to 9.5% (Bacha et al., 2013) and 7 to 12% (Halasz and Lasztity, 1991). Alizadeh et al. (2016) determined the concentration of nucleotides to be 0.13% in a wheat-corn DDGS source, but no studies have been conducted to determine nucleotide content in corn DDGS. There is increasing research evidence that diet supplementation with nucleotides for monogastric animals may affect intestinal morphology and function, immune response, composition of intestinal microbiota, liver function and morphology, as well as growth performance (Sauer et al., 2011).
Changes in microbial populations of the gastrointestinal tract
There is much interest in determining and understanding shifts in the gut microbiome of pigs that occur based on diet composition and interventions on swine health and growth performance. However, our current understanding of positive and negative effects of changes in microbial populations in the gastrointestinal tract on metabolism, nutrient utilization and the immune system is unclear. Tran et al. (2012) fed diets containing up to 30% DDGS to weaned pigs and evaluated patterns of change in fecal microbial populations over time using an electrophoretic fingerprinting technique. Results showed that pigs fed the 30% DDGS diet resulted in a more homogeneous microbial population with fewer bacterial species, but there were no changes on serum immunoglobulin (IgG and IgA) concentrations. These researchers suggested that less microbial diversity in the gut microbiome may be associated with increased microbial ecosystem instability, but there is no direct evidence to support this.
Ewing and Cole (1994) suggested that a greater ratio of Lactobacillus to Enterobacteriaceae spp. could be considered as an index of more favorable gut health conditions because Lactobacillus functions as a major component for prevent infections while Enterobacteriaceae (including Escherichia coli) are detrimental to gut health. Yang et al. (2010) determined the effects of feeding corn DDGS, wheat DDGS and a corn-wheat DDGS source on bacterial profiles in digesta collected at the terminal ileum of pigs. Their results showed that feeding the corn DDGS diet increased Enterobacteriaceae and tended to increase number of Lactobacillus compared with feeding the wheat and corn-wheat DDGS sources. However, the Lactobacillus:Enterobacteriaceae was greater in digesta of pigs fed the wheat DDGS compared with corn DDGS, but the lactic acid concentration was greater from feeding corn DDGS versus wheat DDGS. These researchers suggested that an increase in lactic acid production may potentially inhibit pathogen growth, but provided no direct evidence to support this.
Intestinal cell differentiation and immune response
The small intestine contains many types of cells that have different functions. Enterocytes are involved in nutrient absorption while other cells (i.e. goblet, endocrine, Paneth, tuft and M-cells) have secretory functions. Therefore, understanding the role of different dietary fiber sources (e.g. DDGS) on changes in intestinal cell composition, nutrient transporters and receptors, cell differentiation and the immune system is necessary to improve nutritional efficiency and gut health of pigs. Saqui-Salces et al. (2017) fed a corn-soybean meal diet, or diets containing similar NDF content (about 21%) provided by wheat straw, DDGS or soybean hulls to finishing pigs for 14 days. Their results showed that feeding diets containing DDGS and wheat straw had a greater effect on modulating intestinal cell differentiation by promoting goblet cells and altering expression of nutrient receptors and transporters, than the diets containing soybean hulls or corn-soybean meal.
In a subsequent study, Vila et al. (2018) showed that feeding diets containing DDGS or wheat middlings increased the expression of MUCIN 2 (mucin) in the ileum, without affecting the proportion of goblet cells, compared to feeding a corn-soybean meal diet. They also evaluated ileal gene expression of 12 cytokines to determine if feeding these high-fiber diets, with or without non-starch polysaccharide degrading enzymes, changed the pro-inflammatory and anti-inflammatory responses. There were no effects of feeding DDGS or wheat midds diets on expression of IFNγ, TNFα, IL-1β, IL-2, IL-6, IL-8, IL-10, IL-12p40 and IL-23. These responses were different than those reported by Weber et al. (2008) who showed that feeding a 7.5% DDGS diet for seven days to nursery pigs increased ileal gene expression of IL-6, IL-1β and IL-10. However, the addition of enzymes to DDGS and wheat midds diets did affect the local immune profile of the ileum and favored a pro-inflammatory response. More efforts are needed to study the many complex responses from feeding DDGS diets on functional changes in gastrointestinal tract relative to nutrient utilization and the immune system.
Effects of feeding DDGS under pathogen challenges
Lawsonia intracellularis
There is some evidence that feeding DDGS to swine has beneficial effects on gut health of pigs infected with Lawsonia intracellularis. Whitney et al. (2006a,b) conducted two studies to evaluate the effects of feeding DDGS diets to growing pigs under a moderate L. intracellularis disease challenge. In the first study, pigs were fed diets containing zero or 10% DDGS, with and without antimicrobials (bacitracin methylene disalicylate and pulse dosing of chlortetracycline), for four weeks before infection and 21 days after infection (Whitney et al, 2006a). Feeding the DDGS diet to challenged pigs decreased the prevalence, length and severity of lesions in the ileum and colon, and pigs fed the antimicrobial also had lower prevalence and severity of lesions. Although length, severity and prevalence of lesions were not affected in challenged pigs fed the DDGS diet with antimicrobials, fecal shedding of L. intracellularis was decreased by 14 days post-challenge. These results suggest that DDGS may provide similar benefits to the antimicrobial used in this study for growing pigs challenged with this common pathogen.
However, in a subsequent study, Whitney et al. (2006b) evaluated the effects of feeding diets containing 20% DDGS, 5% soybean hulls or soybean hulls sprayed with a polyclonal antibody product to pigs challenged with L. intracellularis. In this study, lesion length, severity, prevalence and fecal shedding were generally unaffected by diet, but lesion length and severity tended to be less in pigs fed the DDGS diet compared with the diet containing the L. intracellularis polyclonal antibody product. Feeding the DDGS diet had no effect on serum immunoglobulin concentrations compared with challenged pigs fed the other dietary treatments. The mechanisms of these responses are unknown because there is limited information on how feeding DDGS may affect the intestinal microbiota of pigs and their susceptibility to infection or colonization with pathogens.
Salmonella
Rostagno et al. (2013) conducted two experiments to determine if diets containing 20, 30 or 40% DDGS affected susceptibility, intestinal levels and shedding of Salmonella. In one of these experiments, pigs infected with Salmonella and fed the control diet without DDGS, had higher Salmonella shedding frequency than pigs fed the 30% DDGS diet, but the overall responses suggest that diets containing DDGS do not alter the susceptibility to Salmonella colonization in growing-finishing pigs.
Brachyspria
Brachyspira spp. are comprised of a diverse group of Gram-negative spirochetes, of which Brachyspira hyodysenteriae, is known to cause swine dysentery in growing-finishing pigs. In addition, B. hampsonii can also cause disease similar to B. hyodysenteriae, with increased mucosal thickening, hemorrhage and large amounts of mucus in the large intestine. Wilberts et al. (2014) hypothesized that the high insoluble fiber content in DDGS may enhance the intestinal environment to encourage infection from pathogenic Brachyspira spp. and cause clinical colitis. To test this hypothesis, 4-week old pigs were inoculated with an uninfected dose or inoculum containing B. intermedia, B. pilosicoli, B. hampsonii or B. hyodysenteriae, and fed diets containing zero or 30% DDGS for two weeks prior to inoculation through 21 days post-inoculation. Results from this study showed that although feeding DDGS may increase the incidence of swine dysentery, it may not affect the severity. It appears that feeding a 30% DDGS diet may increase the risk of pigs develop swine dysentery, and when exposed to these pathogens, may shed the pathogen one day earlier and develop dysentery almost twice as fast as those fed the 0% DDGS diet.
Risk of virus transmission
Foreign animal diseases, such as foot-and-mouth disease and classical swine fever have had devastating impacts on global food animal production, trade and economics (Yang et al., 1999; Huang et al., 2000; Stegeman et al., 2000; Thompson et al., 2002; Waage et al., 2008). The introduction of porcine epidemic diarrhea virus into the U.S. pork industry in 2013 resulted in a loss of 7 million pigs representing 10% of annual production (Schulz and Tonsor, 2015). Although the specific cause of PEDV introduction has not been conclusively determined, contaminated feed and feed ingredients can serve as sources of transmission of PEDV (Dee et al., 2014) as well as other corona viruses (transmissible gastroenteritis virus; porcine delta corona virus; Trudeau et al., 2017). Dee et al. (2015) showed that PEDV was detected by virus isolation or a bioassay up to 30 days post-inoculation from soybean meal, DDGS, meat and bone meal, red blood cells, lysine HCl, DL methionine, choice white grease, choline chloride and complete feed. Although PEDV survival varied among feed ingredients, this virus appears to survive the longest in soybean meal, but applying a formaldehyde-based liquid treatment can be effective for causing virus inactivation in all ingredients. Similarly, Trudeau et al. (2017a) evaluated survival of PEDV, TGEV and PDCoV in various feed ingredients and also showed that PEDV survived the longest, and TGEV and PDCoV also had high survival in soybean meal compared to several other ingredients, including DDGS. These results suggest that soybean meal is a greater risk factor for transmission of corona viruses via feed than DDGS and other common feed ingredients. Furthermore, thermal treatment at temperatures greater than 70 degrees C inactivated PEDV in complete feed, premix and all ingredients, including DDGS (Trudeau et al., 2017b).
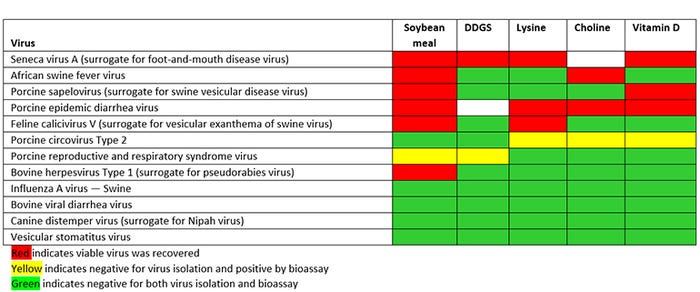
Currently, there is significant concern about the risk of transmission of ASF Fever virus through imported feed ingredients into the United States and other countries (Guinat et al., 2016). If ASFV were to enter the United States, it has been estimated that economic losses may be about $16.5 billion during the first year of an outbreak (Hayes et al., 2011). Therefore, Dee et al. (2018) conducted a study to determine the survival of 11 viral pathogens of global significance, in feed ingredients under simulated transportation times and environmental conditions across the Pacific (37 days) and Atlantic (30 days) oceans to Des Moines, Iowa (Table 2). Surrogate viruses with similar characteristics were used for Seneca virus A, porcine sapelovirus, feline calicivirus v, bovine herpes virus Type 1 and canine distemper virus. Ingredient samples were evaluated using polymerase chain reaction, virus isolation and/or swine bioassays to determine virus survival. Of the five ingredients evaluated, seven viruses survived in soybean meal while only two viruses (Seneca virus A and porcine respiratory and reproductive syndrome virus) survived in DDGS. Furthermore, four viruses survived in lysine, three survived in choline and four viruses survived in vitamin D. ASF virus only survived in soybean meal and choline, but not in DDGS. These initial results suggest that DDGS is a lower risk ingredient for transmission of most of the important viruses, and ASF virus does not appear to survive in DDGS under the environmental conditions and travel times used in this study.
Conclusion
Our knowledge about the potential health benefits and limitations from feeding DDGS diets to pigs is limited. However, it appears that the relatively high antioxidant compounds and capacity of DDGS may be effective for minimizing oxidative stress in pigs without the need for supplemental vitamin E. Components (β-glucan, mannans, nucleotides) of yeast cell walls may also provide some gut health and immune system benefits, but no studies have been conducted to directly evaluate their potential contributions.
Furthermore, more studies are needed to improve our understanding of the effects of DDGS on intestinal cell composition, nutrient transporters and receptors, cell differentiation, immune system, microbiome and metabolomics. This is critical information to potentially modify diet composition to avoid negative effects from specific pathogen challenges, as well as use DDGS under disease challenge conditions when it may provide some beneficial effects. Finally, some viruses (PEDV, TGEV, PDCoV, Seneca virus A and PRRS virus) appear to survive in DDGS, but the risk of transmission of these viruses is much less than that for soybean meal.
References
Alizadeh, M., J.C. Rodriguez-Lecompte, A. Rogiewicz, R. Patterson, and B.A. Slominski. 2016. Effect of yeast-derived products and distillers dried grains with solubles on growth performance, gut morphology, and gene expression of pattern recognition receptors and cytokines in broiler chickens. Poult. Sci. 95:507-517.
Bacha, U., M. Nasir, M.A. Ali, J. Muhammad, and A.A. Sheikh. 2013. Review article: Nucleotides supplementation improves various function of the body. J. Anim. Health and Prod. 1:1-5.
Belyea, R.L., K.D. Rausch, and M.E. Tumbleson. 2004. Composition of corn and distillers dried grains with solubles from dry grind ethanol processing. Bioresource Technol. 94:293-298.
Dee, S.A., F.V. Bauermann, M.C. Niederwerder, A. Singrey, T. Clement, M. de Lima, C. Long, G. Patterson, M.A. Sheahan, A.M.M. Stoian, V. Petrovan, C.K. Jones, J. De Jong, J. Ji, G.D. Spronk, L. Minion, J. Christopher-Hennings, J.J. Zimmerman, R.R.R. Rowland, E. Nelson, P. Sundberg, and D.G. Diel. 2018. Survival of viral pathogens in animal feed ingredients under transboundary shipping models. PLoS ONE 13(3):e0194509. https://doi.org/10.1371/journal.pone.0194509
Dee, S. C. Neill, T. Clement, A. Singrey, J. Christopher-Hennings, and E. Nelson. 2015. An evaluation of porcine epidemic diarrhea virus survival in individual feed ingredients in the presence or absence of a liquid antimicrobial. Porcine Health Mgmt. 1:9.
Dee, S., T. Clement, A. Schelkopf, J. Nerem, D. Knudson, J. Christopher-Hennings, and E. Nelson. 2014. An evaluation of contaminated complete feed as a vehicle for porcine epidemic virus infection of näive pigs following consumption via natural feeding behavior: Proof of concept. BMC Vet. Res. 10:176. https://doi.org/10.1186/s12917-014-0176-9
Ewing, W.N., and D.J.A. Cole. 194. The Living Gut. An Introduction to Microorganisms in Nutrition Context. Context graphics, Dungannon, Ireland, UK.
Guinat, C., A. Gogin, S. Biome, G. Keil, R. Pollin, D.U. Pfeiffer, and L. Dixon. 2016. Transmission routes of African swine fever virus to domestic pigs: current knowledge and future research directions. Vet. Rec. 178:262-267.
Halasz, A., and R. Lasztity. 1991. Use of yeast biomass in food production. CRC Press, Inc., Boca Raton, FL. Pp. 23-44.
Han, J., and K. Liu. 2010. Changes in composition and amino acid profile during dry grind ethanol processing from corn and estimation of yeast contribution toward DDGS proteins. J. Agric. Food Chem. 58:3430-3437.
Hanson, A.R., P.E. Urriola, L.J. Johnston, and G.C. Shurson. 2015a. Impact of synthetic antioxidants on lipid peroxidation of distillers dried grains with solubles and distillers corn oil in extreme temperature and humidity conditions. J. Anim. Sci. 93:4070-4078.
Hanson, A.R., L.J. Johnston, S.K. Baidoo, J. Torrison, C. Chen, and G.C. Shurson. 2015b. Effect of feeding peroxidized dried distillers grains with solubles to sows and progeny on growth performance and metabolic oxidative status of nursery pigs. J. Anim. Sci. 93:135-146.
Hayes, D., J. Fabiosa, A. Elobeid, and M. Carriquiry. 2011. Economy wide impacts of a foreign animal disease in the United States: Working paper 11-WP 525. Center for Agric. Rural Devel., Iowa State Univ., Ames, IA.
Huang, C.C., M.J. Jong, and S.Y. Lin. 2000. Characteristics of the foot-and-mouth disease virus in Taiwan. J. Vet. Med. Sci. 62:677-679.
Hung, Y.T., A.R. Hanson, G.C. Shurson, and P.E. Urriola. 2017. Peroxidized lipids reduce growth performance of poultry and swine: A meta-analysis. Anim. Feed Sci. Technol. 231:47-58.
Ingledew W.M. 1999. Yeast — could you base business on this bug? Pages 27-47 in T.P. Lyons and K. A.Jacques, editors. Under the microscope — focal points for the new millennium — biotechnology in the feed industry. Proc. Alltech’s 15th Annual Symposium. Nottingham University Press, Nottingham, UK.
Kerr, B.J., T.A. Kellner, and G.C. Shurson. 2015. Characteristics of lipids and their feeding value in swine diets. J. An. Sci. Biotech. 6:30.
Kim, Y., N.S. Mosier, R. Hendrickson, T. Ezeji, H. Blaschek, B. Dien, M. Cotta, B. Dale, and M.R. Landisch. 2008. Composition of corn dry-grind ethanol by-products: DDGS, wet cake, and thin stillage. Bioresour. Technol. 99:5156-5176.
Li, G., X. Wang, M. Lin, Z. Lu, and W. Yao. 2012. Effects of corn DDGS in combination with compound enzymes on growth performance, carcass fat quality, and plasma and tissue homeostasis of growing-finishing pigs. Livestock Sci. 149:46-52.
Lim C., M. Yildirim-Aksoy, and P.H. Klesius. 2009. Growth response and resistance to Edwardsiella ictaluri of channel catfish, Ictalurus punctatus, fed diets containing distillers dried grains with solubles. J. World Aquacult. Soc. 40:182-193.
Luthria, D.L., A.A. Memon, and K. Liu. 2014. Changes in phenolic acid content during dry-grind processing of corn into ethanol. J. Sci. Food Agric. 94:1723-1728.
Luthria, D.L., K. Liu, and A.A. Memon. 2012. Phenolic acids and antioxidant capacity of distillers dried grains with solubles as compared with corn. J. Am. Oil Chem. Soc. Doi: 10.1007/s11746-012-2025-y
Rop, O., J. Mlcek, and T. Jurikova. 2009. Beta-glucans in higher fungi and their health effects. Nutr. Rev. 67:624-631.
Rostagno, M.H., B.T. Richert, L.V.C. Girao, G.M. Preis, L.J. Lara, A.F. Amaral, A.D.B. Melo, and A. Jones. 2013. Do distillers grains with solubles affect the occurrence of Salmonella enterica colonization in pigs? J. Anim. Sci. 91(E-Suppl. 2): 699 (Abstr.).
Saeed, S., J. Qunitin, H.H.D. Kerstens, N.A. Rao, A. Aghajanirefah, F. Matarese, S.C. Chng, J. Ratter, K. Berentsen, M.A. van der Ent, N. Sharifi, E.M. Janssen-Megens, M.T. Huurne, A. Mandoli, T. van Schaik, A. Ng, F. Burden, K. Downes, M. Frontini, V. Kumat, E.J. Giamarellos-Bourboulis, W.H. Ouwehand, J.W.M. van der Meer, L.A.B. Joosten, C. Wijmenga, J.H.A. Martens, R.J. Xavier, C. Logie, M.G. Netea, and H.G. Stunnenberg. 2014. Epigenetic programming of monocyte-macrophage differentiation and trained innate immunity. Science 345:1251086.
Sauer, N., R. Mosenthin, and E. Bauer. 2011. The role of dietary nucleotides in single-stomached animals. Nutr. Res. Rev. 24:46-59.
Saqui-Salces, M, Z. Huang, M. Ferrandis Vila, J. Li, J.A. Mielke, P.E. Urriola, and G.C. Shurson. 2017. Modulation of intestinal cell differentiation in growing pigs is dependent on the fiber source in the diet. J. Anim. Sci. 95:1179-1190.
Schulz, L.L., and G.T. Tonsor. 2015. Assessment of the economic impacts of porcine epidemic diarrhea virus in the United States. J. Anim. Sci. 93:5111-5118.
Shin, E.-C., G.C. Shurson, and D.D. Gallaher. 2018. Antioxidant capacity and phytochemical content of 16 sources of corn distillers dried grains with solubles. Anim. Nutr. 4:435-441.
Shurson, G.C. 2018. Yeast and yeast derivatives in feed additives and ingredients: Sources, characteristics, animal responses, and quantification methods. Anim. Feed Sci. Technol. 235:60-76.
Shurson, G.C., B.J. Kerr, and A.R. Hanson. 2015. Evaluating the quality of feed fats and oils and their effects on pig growth performance. J. Anim. Sci. and Biotech. 6:10.
Sikora, P., S.M. Tosh, Y. Brummer, and O. Olsson. 2013. Identification of high β-glucan oat lines and localization and chemical characterization of their seed kernel β-glucans. Food Chem. 137:83-91.
Song, R., C. Chen, L.J. Johnston, B.J. Kerr, T.E. Weber, and G.C. Shurson. 2014. Effects of feeding diets containing highly peroxidized distillers dried grains with solubles and increasing vitamin E levels to wean-finish pigs on growth performance, carcass characteristics, and pork fat composition. J. Anim. Sci. 92:198-210.
Song, R., and G.C. Shurson. 2013. Evaluation of lipid peroxidation level in corn dried distillers grains with solubles. J. Anim. Sci. 91:4383-4388.
Song, R., C. Chen, L. Wang, L.J. Johnston, B.J. Kerr, T.E. Weber, and G.C. Shurson. 2013. High sulfur content in corn dried distillers grains with solubles protects against oxidized lipids by increasing sulfur-containing antioxidants in nursery pigs. J. Anim. Sci. 91:2715-2728.
Spring, P, C. Wenk, A. Connolly, and A. Kiers. 2015. A review of 733 published trials on Bio-Mos, a mannan oligosaccharide, and Actigen, a second-generation mannose rich fraction, on farm and companion animals. J. Appl. Anim. Nutr. 3:1-11.
Stegeman, A., A. Elbers, H. deSmit, H. Moser, J. Smak, and F. Pluimers. 2000. The 1997-1998 epidemic of classical swine fever in the Netherlands. Vet. Microbiol. 13:183-186.
Thompson, D., P. Muriel, D. Russell, P. Osborne, A. Bromley, M. Rowland, S. Creigh-Tyte, and C. Brown. 2002. Economic costs of foot and mouth disease outbreak in the United Kingdom in 2001. Rev. Sci. Tech. 21:657-687.
Tran, H., R. Moreno, E.E. Hinkle, J.W. Bundy, J. Walter, T.E. Burkey, and P.S. Miller. 2012. Effect of corn distillers dried grains with solubles on growth performance and health status indicators in weanling pigs. J. Anim. Sci. 90:790-801.
Trudeau, M.P., H. Verma, F. Sampedro, P.E. Urriola, G. C. Shurson, and S.M. Goyal. 2017a. Environmental persistence of porcine coronaviruses in feed and feed ingredients. PLoS ONE 12:e0178094. https://doi.org/10.1371/journal.pone.0178094.
Trudeau, M.P., H. Verma, P.E. Urriola, F. Sampedro, G.C. Shurson, and S.M. Goyal. 2017b. Survival of porcine epidemic diarrhea virus in thermally treated feed ingredients and on surfaces. Porcine Health Management 3:17.
Vetvicka, V., L. Vannucci, and P. Sima. 2014. The effects of β-glucan on pig growth and immunity. The Open Biochem. J. 8:89-93.
Vila, M.F., M.P. Trudeau, Y.-T. Hung, Z. Zeng, P.E. Urriola, G.C. Shurson, and M. Saqui-Salces. 2018. Dietary fiber sources and non-starch polysaccharide-degrading enzymes modify mucin expression and the immune profile of the swine ileum. PLoS ONE 13(11): e0207196. https://doi.org/10.1371/journal.pone.0207196
Waage, J.K., and J.D. Mumford. 2008. Agricultural biosecurity. Phil. Trans. R. Soc. B. 363:863-876.
Weber, T.E., and B.J. Kerr. 2011. Effect of dietary distillers dried grains with solubles on indicators of oxidative stress and immune function in growing pigs. Livest. Sci. 142:85-91.
Weber, T.E., C.J. Ziemer, and B.J. Kerr. 2008. Effects of adding fibrous feedstuffs to the diet of young pigs on growth performance, intestinal cytokines, and circulating acute-phase proteins. J. Anim. Sci. 86:871-881.
Whitney, M.H., G.C. Shurson, and R.C. Guedes. 2006a. Effect of dietary inclusion of distillers dried grains with solubles on the ability of growing pigs to resist a Lawsonia intracellularis challenge. J. Anim. Sci. 2006. 84:1860-1869.
Whitney, M.H., G.C. Shurson, and R.C. Guedes. 2006b. Effect of including distillers dried grains with solubles in the diet, with or without antimicrobial regimen, on the ability of growing pigs to resist a Lawsonia intracellularis challenge. J. Anim. Sci. 2006. 84:1870-1879.
Wilberts, B.L., P.H. Arruda, J.M. Kinyon, T.S. Frana, C. Wang, D.R. Magstadt, D.M. Madson, J.F. Patience, and E.R. Burrough. 2014. Investigation of the impact of increased dietary insoluble fiber through the feeding of distillers dried grains with solubles on the incidence and severity of Brachyspira-associated colitis in pigs. PLoS ONE 9(12): e114741.
Winkler, J.K., K.A. Rennick, F.J. Eller, and S.F. Vaughn. 2007. Phytosterol and tocopherol components in extracts of corn distillers dried grain. J. Agric. food Chem. 55:6482-6486.
Winkler-Moser, J.K., and L. Breyer. 2011. Composition and oxidative stability of crude oil extracts of corn germ and distillers grains. Ind. Crops Prod. 33:572-578.
Yang, P.C., R.M. Chu, W.B. Chung, and H.T. Sung. 1999. Epidemiological characteristics and financial costs of the 1997 foot-and-mouth disease epidemic in Taiwan. Vet. Rec. 145:731-734.
Yang, Y., E. Kiarie, B.A. Slominski, A. Brûlé-Babel, and C.M. Nyachoti. 2010. Amino acid and fiber digestibility, intestinal bacterial profile, and enzyme activity in growing pigs fed dried distillers grains with solubles-based diets. J. Anim. Sci. 88:3304-3312.
Source: Jerry Shurson, who is solely responsible for the information provided, and wholly owns the information. Informa Business Media and all its subsidiaries are not responsible for any of the content contained in this information asset.
You May Also Like



