Reaching an understanding of fiber characteristics of corn DDGS
Part 5 in the series “What have we learned about feeding DDGS to pigs over the past 20 years?”
November 16, 2018

By Jerry Shurson and Pedro Urriola, University of Minnesota Department of Animal Science
The gross energy content of corn distiller’s dried grains with solubles (5,429 kilocalories per kilogram DM) is much greater than corn (4,454 kcal/kg DM), soybean meal (4,730 kcal/kg DM), and other common ingredients used in swine diets. However, the efficiency of pigs to utilize GE in DDGS, as measured by the net energy-to-GE ratio, is low (0.49) compared with that in corn (0.68; NRC, 2012), but the NE:GE can vary from 0.42 to 0.46 among DDGS sources with variable crude fat content (Kerr et al., 2015).
Of all of the chemical components in feed ingredients, lipids provide the greatest amount of NE (kcal/kg), followed by crude protein and starch, with a limited amount provided from dietary fiber (Noblet and van Milgen, 2004). Therefore, the low starch content (0.84 to 3.89%, Kerr et al., 2013; 5.2 to 11.4%, Urriola et al., 2010) and relatively high neutral detergent fiber content (30.2 to 39.7%; Pedersen et al., 2014) in corn DDGS, are the primary reasons for the relatively low GE:NE.
Related: 20 years of DDGS lessons in pig diets
To improve caloric and nutritional efficiency when feeding DDGS diets to pigs, we need to gain a better understanding of the chemical and physical characteristics that affect the digestibility and fermentability of fiber, and explore approaches to convert a greater proportion of these calories to NE in pigs.
Installments in the DDGS series
Part 1: 20 years of DDGS lessons in pig diets
Part 2: Varied energy and digestible amino acids levels in DDGS manageable
Part 3: Work continues to evaluate performance responses from feeding DDGS
Part 4: Managing carcass yield, pork fat quality when feeding corn DDGS
Part 5: Reaching an understanding of fiber characteristics of corn DDGS
Part 6: Enzymes, pre-treatment improve fiber and nutrient digestibility
Part 7: DDGS show greater antioxidant capacity than in corn grain
Fiber content in feed ingredients is measured using several different chemical methods including crude fiber, NDF, acid detergent fiber and total dietary fiber which is comprised of insoluble dietary fiber and soluble dietary fiber, as well as non-starch polysaccharides, that quantify different types and proportions of indigestible carbohydrates, but may not adequately relate directly to nutritional and physiological effects in the animal (Figure 1). The most common measures that have been used to characterize the fiber content in DDGS are NDF, ADF and TDF (which can be separated into IDF and SDF fiber fractions). The NDF, ADF and hemicellulose (determined by difference between NDF and ADF) content (dry matter basis) of corn DDGS sources ranges from 28.8 to 44.0%, 9.0 to 14.0%, and 18.5 to 30.0%, respectively (Kerr et al., 2013).
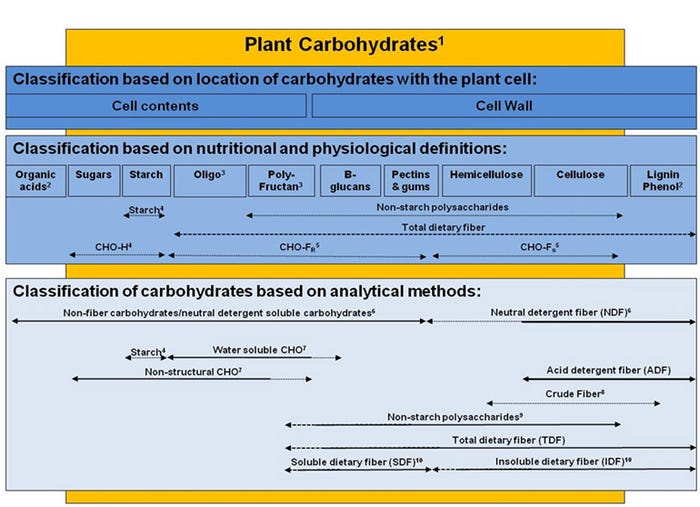
The apparent total tract digestibility of NDF has been reported to range from 44.5 to 61.5% among 15 corn DDGS sources (Kerr et al., 2013). The range in TDF content (dry matter basis) among DDGS sources appears to be similar among studies, where Kerr et al. (2013) reported a range from 30.8 to 37.8% TDF and Urriola et al. (2010) reported a range from 32.9 to 38.6%. However, most of the TDF in DDGS is insoluble (31.8 to 37.3%), with minimal amounts of soluble fiber (0.0 to 1.8%; Urriola et al. 2010).
The most comprehensive study to help us achieve a better understanding of the digestibility and fermentability of fiber in DDGS was conducted by Urriola et al. (2010). Results from three experiments showed that the ATTD of TDF ranged from 29.3 to 57.0% among corn DDGS sources. They also determined the apparent ileal digestibility, apparent total tract digestibility and hindgut fermentability of ADF, NDF, IDF, SDF and TDF by feeding eight corn DDGS sources to growing pigs (Table 1). The fiber digestibility and fermentability varied substantially among DDGS sources regardless of fiber measure used. This variability may be partially attributed to the amounts and types of enzymes, as well as different production processes used in ethanol and co-product production facilities to produce DDGS. Furthermore, about 46% of NDF and 29% of TDF is digestible in the small intestine, while about 13% of NDF and 21% of TDF is fermented in the hindgut. These proportions of digestible and fermentable carbohydrates are assumed to contribute to the total ME and NE content of DDGS, and the wide range in digestibility and fermentability among sources may partially contribute to the range in ME and NE content of DDGS reported in many studies. However, the AID of IDF is much less than SDF, and IDF is less fermentable than SDF in the hindgut. Although the average ATTD of NDF (59%) and TDF (50%) are moderately high in corn DDGS compared with some other types of high-fiber ingredients, there is a tremendous need to explore ways of improving AID and hind gut fermentation of the high proportion of IDF in DDGS to enhance the NE content.

Furthermore, the physical structure of fiber in corn DDGS may trap some of the oil in DDGS, prevent it from being accessible to digestive enzymes, and contribute to the relatively low GE:ME and GE:NE content. In fact, Kerr et al. (2013) reported that ATTD of ether extract (oil) ranged from 53 to 81% among corn DDGS sources. Therefore, it is likely that the combined differences in fiber digestibility and fermentability, along with the variability of lipid digestibility involving fiber structure, are the primary reasons for variable ME and NE content among DDGS sources fed to swine.
Although it is useful to understand the differences in digestibility and fermentability of ADF, NDF, IDF and SDF in DDGS, knowledge of the NSP composition of DDGS is extremely important when selecting commercially available feed enzymes to improve energy and nutrient digestibility of DDGS in pigs. Pedersen et al. (2014) determined the NSP composition (dry matter basis) of 47 corn DDGS samples and showed that NSP’s represent about 25 to 34% of the total composition, of which most NSP’s are insoluble (Table 2). Cellulose represents about 5 to 9% of corn DDGS content, and the predominant non-cellulosic polysaccharides are xylose (7.7%) and arabinoxylose (12.3 to 17.2%), which are mainly insoluble. The mannose content in corn DDGS (1.7%) is substantially greater than found in corn grain, and is likely due to the mannan content in residual yeast cell walls that are present in DDGS (Shurson, 2017).

Corn DDGS has a relatively high arabinose (6.2%) and uronic acid (1.6%) content, which results in relatively high arabinose to xylose, and uronic acid-to-xylose ratios. This indicates that the fiber (heteroxylan) structure is complex and variable in corn DDGS, and therefore, is more difficult to degrade with exogenous enzymes than corn grain. Klason lignin is not a well-defined chemical constituent, and may contain protein (Maillard products), residual lipids and waxes, and cutin in addition to true lignin. However, Klason lignin represents 1.5 to 4.7% of the chemical composition of DDGS. These results suggest that the concentrations of substituted xylan and soluble NSPs in DDGS are altered during the production process and are substantially different from their original structure in corn grain.
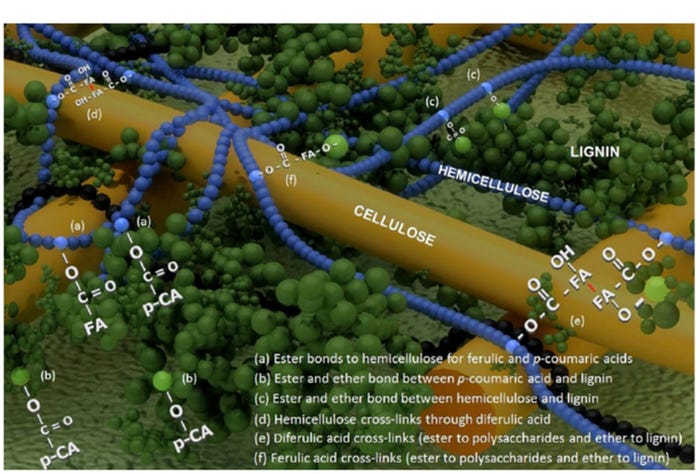
We also need to achieve a better understanding of the physical structure of fiber, and identify practical and effective methods to degrade this structure, to improve the effectiveness of enzymes and the utilization of energy and nutrients in DDGS. The primary cell wall structure in cereal grains is comprised of a skeleton of cellulosic microfibrils embedded in a matrix of hemicelluloses and smaller amounts of pectins, glycoproteins and hydroxycinnamates.
Subsequently, as the secondary cell wall develops during grain maturation, p-coumaryl, coniferyl and sinapyl alcohols are co-polymerized to form mixed lignins (Santiago et al., 2013). The addition of mixed lignins to the cell wall structure provides added strength to the fiber structure and causes it to be resistant to degradation.
In corn grain, the most abundant hemicelluloses are arabinoxylans, which are comprised of a β (1→4)-d-xylan backbone with substitutions of arabinose, glucuronic acid and acetic acid. The hemicellulose is tangled with cellulose microfibrils by hydrogen bonds (Figure 2). These hydrogen bonds cause the cell wall to be less accessible to degradation (Somerville et al., 2004). However, this implies that the removal of arabinoxylans from the surface region of fiber by the addition of xylanases, can result in exposure of cellulose microfibrils (crystalline structure) which are highly resistant to acids and enzymatic hydrolysis (Hall et al., 2010). In fact, Pedersen et al. (2015) reported that the AID (11.9%) and ATTD (29.0%) of cellulose in wheat DDGS for swine, is less than AID (37%) and ATTD (43.8%) in other fiber components. Therefore, it is possible that the more stable cellulosic microfibrils embed or trap arabinoxylans in corn DDGS, resulting in decreased apparent total tract digestibility of fiber and prevent xylanase from accessing its substrates.
Furthermore, understanding the changes in morphology of fiber before and after the degradation processes may be useful in identifying approaches to improve the utilization of fiber in DDGS for pigs. Results from several studies have shown that crystalline celluloses are much more resistant to enzymatic hydrolysis compared to those with low crystallinity (Fan et al., 1980; Zhang and Lynd, 2004; Hall et al., 2010). In addition, the crystallinity and crystal size of natural fiber sources have been shown to increase during thermal processing (Poletto et al., 2014). It is well known that the production of DDGS involves drying temperatures greater than 100 C as it exits the dryer (Rosentrater et al., 2012). This may indicate that the most readily degradable fiber may have already been partially degraded during DDGS production and thus, limits the effectiveness of feed enzymes or other processing technologies in diets containing DDGS.
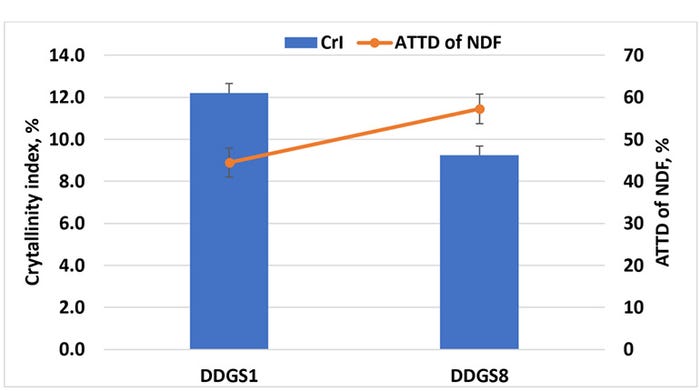
We have initiated studies to assess the role of the physical structure on digestibility of fiber in corn DDGS using X-ray diffraction to measure the extent of crystallization of two sources of DDGS with low (44.5%) and high (57.3%) ATTD of NDF for swine (Kerr et al., 2013). Our initial results showed that the source of DDGS with less ATTD of NDF had a greater crystallinity index than the source of DDGS with high ATTD of NDF (Figure 3). Therefore, increasing degradability of dietary fiber in DDGS may require disruption of the crystalline structure of fiber in DDGS (Urriola et al., 2018).
References
Fan, L.T., Y.H. Lee, and D.H. Beardmore. 1980. Mechanism of the enzymatic-hydrolysis of cellulose — effects of major structural features of cellulose on enzymatic-hydrolysis. Biotechnol. and Bioengineer. 22:177-199.
Hall, M., P. Bansal, J.H. Lee, M.J. Realff, and A.S. Bommarius. 2010. Cellulose crystallinity — a key predictor of the enzymatic hydrolysis rate. FEBS J. 277:1571-1582.
Kerr, B.J., W.A. Dozier, III, and G.C. Shurson. 2013. Effects of reduced-oil corn distillers dried grains with solubles composition on digestible and metabolizable energy value and prediction in growing pigs. J. Anim. Sci. 91:3231-3243.
Kerr, B.J., N.K. Gabler, and G.C. Shurson. 2015. Compositional effects of corn distillers dried grains with solubles with variable oil content on digestible, metabolizable, and net energy values in growing pigs. Prof. Anim. Scientist 31:485-496.
Noblet, J., and J. van Milgen. 2004. Energy value of pig feeds: Effect of pig body weight and energy evaluation system. J. Anim. Sci. 82(E. Suppl.):E229-E238.
NRC. 2007. Nutrient Requirements of Horses. 6th rev. ed. Natl. Acad. Press, Washington, D.C., p. 206.
NRC. 2012. Nutrient requirements of swine. 11th rev. ed. Natl. Acad. Press, Washington, DC.
Pedersen, M.B., S. Dalsgaard, K.E. Bach Knudsen, S. Yu, and H.N. Lærke. 2014. Compositional profile and variation of distillers dried grains with solubles from various origins with focus on non-starch polysaccharides. Anim. Feed Sci. Technol. 197:130-141.
Pedersen, M.B., S. Yu, S. Arent, S. Dalsgaard, K.E. Bach Knudsen, and H.N. Laerke. 2015. Xylanase increased the ileal digestibility of nonstarch polysaccharides and concentration of low molecular weight nondigestible carbohydrates in pigs fed high levels of wheat distillers dried grains with solubles. J. Anim. Sci. 93:2885-2893.
Poletto, M., H. Júnior, and A. Zattera. 2014. Native cellulose: Structure, characterization and thermal properties. Materials 7:6105-6119.
Rosentrater, K.A., K. Ileleji, and D.B. Johnson. 2012. Manufacturing of fuel ethanol and distillers grains — current and evolving processes. In: Liu K, Rosentrater K. A and B. Raton (eds.) Distillers grains production, properties, and utilization. p. 73-102.
Santiago, R., J. Barros-Rios, and R.A. Malvar. 2013. Impact of cell wall composition on maize resistance to pests and diseases. Int. J. Mol. Sci. 14:6960-6980.
Shurson, G.C. 2017. Review — Yeast and yeast derivatives in feed additives and ingredients: sources, characteristics, animal responses, and quantification methods. Anim. Feed Sci. Technol. 235:60-76.
Somerville, C., S. Bauer, G. Brininstool, M. Facette, T. Hamann, J. Milne, E. Osborne, A. Paredez, S. Persson, T. Raab, S. Vorwerk, and H. Youngs. 2004. Toward a systems approach to understanding plant cell walls. Science 306:2206-2211.
Urriola, P.E., G.C. Shurson, and H.H. Stein. 2010. Digstibility of dietary fiber in distillers coproducts fed to growing pigs. J. Anim. Sci. 88:2373-2381.
Urriola, P. E., Z. K. Zeng, and G. C. Shurson. 2018. Strategies to modify the fiber structure and increase digestible energy content in corn distillers dried grains with solubles. Final Report to the National Bork Board, Des Moines, IA.
Zhang, Y.H., and L.R. Lynd. 2004. Toward an aggregated understanding of enzymatic hydrolysis of cellulose: Noncomplexed cellulase systems. Biotechnol Bioeng 88:797-824.
You May Also Like



