Do extreme weather events increase the risk of PED breaks?
All extreme weather events could also affect workforce availability and absenteeism in the immediate time around the event.
June 15, 2023

Porcine epidemic diarrhea virus was introduced in North America around 2013 [1,2], and since then, the estimated economic losses for PED are estimated to be approximately $150 million dollars a year [3]. Contact with infected animals is the main between-farm transmission mode [4] of PED, as such, the introduction of infected pigs in a farm is associated with an increased risk of the farm having an outbreak [5,6]. However, the movement of personnel, vehicles, fomites [7,8] and the overall biosecurity measures taken on farms [9] are recognized as important risk factors for the disease.
Proximity to positive farms, as well as the farm density in a region [10] have also been shown to be associated with a higher chance of a farm being positive for PED. Farm size [11], which captures the number of animals on the farm as well as other factors that likely scale with number of animals, such as personnel and feed movements, is also associated with PED risk.
Contaminated feed has been hypothesized as a potential risk factor, as PED (and other viruses) has been shown to survive in feed for long periods [12–14], even though it's contribution to overall PED risk seem to be low [15]. Environmental characteristics, such as land use, vegetation type and slope of the terrain around a farm have also been pointed as risk factors for PED [10].
Despite the wealth of knowledge regarding risk factors for the occurrence of PED, those associated with weather have historically received little attention. Extreme weather events may change the exposure of animals to viruses that may be present on the environment either directly (via structural damage to farms, increase in the amount of virus from overflooded manure lagoons and roads) or indirectly (due to disruptions on personnel availability and routes trucks may be forced to do due to the weather event). We hypothesize that extreme weather events may change the risk of PED outbreak on swine farms. As such, our objective is to compare if farms that had PED outbreaks were exposed to extreme weather events more often than control farms.
We conducted a 1:4 case-control study to investigate if the frequency of exposure to extreme weather events was different between cases (farms experiencing new outbreaks of PED) and controls (farms not experiencing a new outbreak of PED). Outbreaks of PED occurring in one swine-dense producing region of the United States between 2014 and 2019 were identified via the Morrison Swine Health Monitoring Project. Extreme weather event data were obtained from the National Oceanic and Atmospheric Administration storm events database. A farm's exposure to a weather event was evaluated based on if a weather event was reported on each farm's county on each of the 10 preceding weeks (lags) to an outbreak (for cases) or to the date in which controls were randomly selected.
In order to account for other factors that could potentially impact the occurrence of PED, we obtained information on various risk factors to control for their influence on PED occurrence. These include details about the farm's production system (companies), type and size, as well as the year and season in which the data was collected (obtained from MSHMP). We also controlled for the farm's altitude, the number of breeding sites within a 5km radius of each farm and for the pig density per county. Association estimates (reported as odds ratios) and their 95% confidence intervals of each weather event on the 10 preceding weeks, controlled for the covariates described above, can be found on Figure 1.
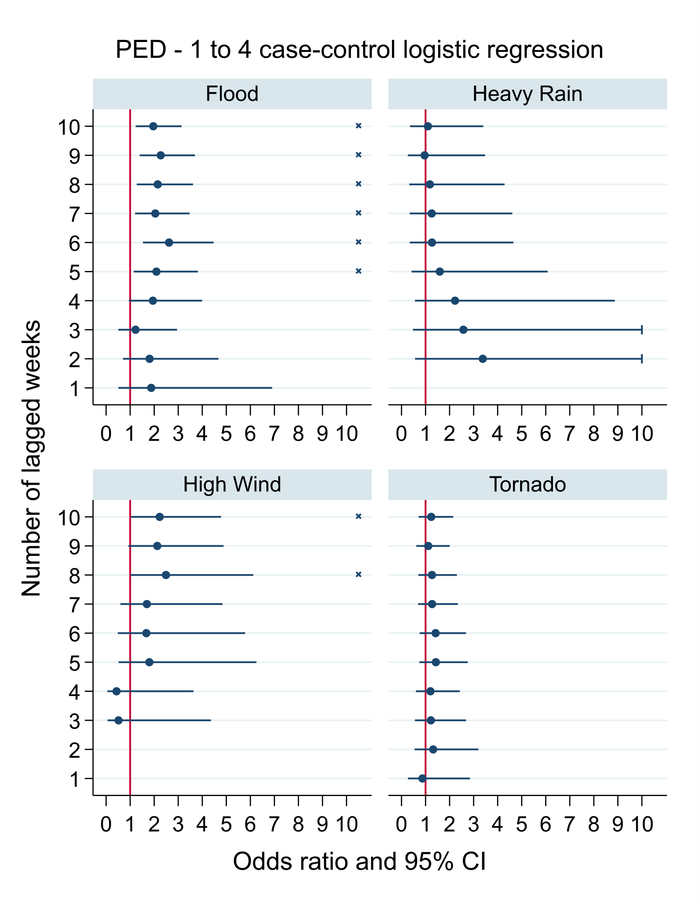
We found that farms situated in a county that had experienced a flooding event five to 10 weeks prior were approximately two to three times more likely to have a PED outbreak compared to controls. Farms situated in a county that experienced high wind events in the eight to 10 preceding weeks were approximately three times more likely to have a PED outbreak compared to controls. We did not find significant associations between the occurrence of heavy rain or tornados and PED outbreaks. Overall, associations tended to be relatively weak and with wide confidence intervals.
The exact mechanism by which each weather event could act to change the risk of disease occurrence was not possible to investigate. Despite this, it is possible to imagine scenarios in which weather events disrupt a farm's biosecurity protocols or create breaches in containment. Floods can cause breaches in lagoons in which manure is kept, for example, which may contaminate roads, trucks and cars used to move animals, feed, or employees moving between farms. The opportunity for biosecurity practices to be overwhelmed in such scenarios could potentially increase. Tornados and high winds could cause more destructive effects, in which roofs, walls or parts of farms could be blown away (thus increasing the environmental output of virus from a positive farm, for example). Additionally, high winds and tornados could also overwhelm a farm's air filtration systems.
All extreme weather events could also affect workforce availability and absenteeism in the immediate time around the event. Schedule and route disruptions for dead animal removal, animal or feed shipments could present challenges when trying to create the most biosecure option, as certain roads may be not drivable or inaccessible. Such changes could arguably be as or even potentially more important than direct structural damage.
We found that flooding and high wind occurrence was associated with an increase in the risk of PED between 5 to 10, and 8 to 10 weeks after the events, respectively. Other weather events potentially have too small of an effect or are not associated with the occurrence of PED. Further studies to investigate the association of weather events and disease occurrence on swine farms should be conducted, particularly if exposure to weather events can be assessed directly on farms, which is a big limitation of our analysis. We suggest that swine-producing companies in the United States should develop biosecurity protocols to account for extreme weather events and diminish the risk those may pose to the introduction of pathogens on their herds.
References
1. Ojkic D, Hazlett M, Fairles J, Marom A, Slavic D, Maxie G, et al. The first case of porcine epidemic diarrhea in Canada. Can Vet J = La Rev Vet Can [Internet]. 2015 Feb;56(2):149–52. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25694663
2. Vlasova AN, Marthaler D, Wang Q, Culhane MR, Rossow KD, Rovira A, et al. Distinct Characteristics and Complex Evolution of PEDV Strains, North America, May 2013–February 2014. Emerg Infect Dis [Internet]. 2014 Oct;20(10). Available from: http://wwwnc.cdc.gov/eid/article/20/10/14-0491_article.htm
3. Thomas P, Coleman K. Impactful investments: Measuring return on investment for biosecurity. In: AASV Annual Meeting [Internet]. Perry, Iowa, USA: American Association of Swine Veterinarians; Available from: https://doi.aasv.org/10.54846/am2023/163
4. Jung K, Saif LJ. Porcine epidemic diarrhea virus infection: Etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet J [Internet]. 2015 May;204(2):134–43. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1090023315000787
5. Bierk MD, Dee SA, Rossow KD, Otake S, Collins JE, Molitor TW. Transmission of porcine reproductive and respiratory syndrome virus from persistently infected sows to contact controls. Can J Vet Res [Internet]. 2001 Oct;65(4):261–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11768134
6. Machado G, Vilalta C, Recamonde-Mendoza M, Corzo C, Torremorell M, Perez A, et al. Identifying outbreaks of Porcine Epidemic Diarrhea virus through animal movements and spatial neighborhoods. Sci Rep [Internet]. 2019 Dec 24;9(1):457. Available from: http://www.nature.com/articles/s41598-018-36934-8
7. VanderWaal K, Deen J. Global trends in infectious diseases of swine. Proc Natl Acad Sci [Internet]. 2018 Nov 6;115(45):11495–500. Available from: http://www.pnas.org/lookup/doi/10.1073/pnas.1806068115
8. Pitkin A, Deen J, Dee S. Further assessment of fomites and personnel as vehicles for the mechanical transport and transmission of porcine reproductive and respiratory syndrome virus. Can J Vet Res. 2009;73(4):298–302.
9. Lowe J, Gauger P, Harmon K, Zhang J, Connor J, Yeske P, et al. Role of Transportation in Spread of Porcine Epidemic Diarrhea Virus Infection, United States. Emerg Infect Dis [Internet]. 2014 May;20(5):872–4. Available from: http://wwwnc.cdc.gov/eid/article/20/5/13-1628_article.htm
10. Kikuti M, Drebes D, Robbins R, Dufresne L, Sanhueza JM, Corzo CA. Growing pig incidence rate, control and prevention of porcine epidemic diarrhea virus in a large pig production system in the United States. Porc Heal Manag [Internet]. 2022 Dec 7;8(1):23. Available from: https://porcinehealthmanagement.biomedcentral.com/articles/10.1186/s40813-022-00268-9
11. Velasova M, Alarcon P, Williamson S, Wieland B. Risk factors for porcine reproductive and respiratory syndrome virus infection and resulting challenges for effective disease surveillance. BMC Vet Res [Internet]. 2012;8(1):184. Available from: http://bmcvetres.biomedcentral.com/articles/10.1186/1746-6148-8-184
12. Kim Y, Yang M, Goyal SM, Cheeran MC-J, Torremorell M. Evaluation of biosecurity measures to prevent indirect transmission of porcine epidemic diarrhea virus. BMC Vet Res [Internet]. 2017 Dec 5;13(1):89. Available from: http://bmcvetres.biomedcentral.com/articles/10.1186/s12917-017-1017-4
13. Evans CM, Medley GF, Green LE. Porcine reproductive and respiratory syndrome virus (PRRSV) in GB pig herds: farm characteristics associated with heterogeneity in seroprevalence. BMC Vet Res [Internet]. 2008;4(1):48. Available from: http://bmcvetres.biomedcentral.com/articles/10.1186/1746-6148-4-48
14. Mortensen S, Stryhn H, Søgaard R, Boklund A, Stärk KDC, Christensen J, et al. Risk factors for infection of sow herds with porcine reproductive and respiratory syndrome (PRRS) virus. Prev Vet Med [Internet]. 2002 Feb 14;53(1–2):83–101. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11821139
15. Paploski IAD, Bhojwani RK, Sanhueza JM, Corzo CA, VanderWaal K. Forecasting viral disease outbreaks at the farm-level for commercial sow farms in the U.S. Prev Vet Med [Internet]. 2021 Nov;196:105449. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0167587721001938
16. Mai TN, Yamazaki W, Bui TP, Nguyen VG, Le Huynh TM, Mitoma S, et al. A descriptive survey of porcine epidemic diarrhea in pig populations in northern Vietnam. Trop Anim Health Prod [Internet]. 2020 Nov 4;52(6):3781–8. Available from: https://link.springer.com/10.1007/s11250-020-02416-1
17. Firkins LD, Weigel RM. A retrospective study of risk factors for porcine reproductive and respiratory syndrome virus infection and clinical disease in swine herds in Illinois during the early years of the pandemic. J Swine Heal Prod [Internet]. 2004;12(1):23–8. Available from: https://www.aasv.org/shap/issues/v12n1/v12n1p23.pdf
18. Trudeau MP, Verma H, Sampedro F, Urriola PE, Shurson GC, Goyal SM. Environmental persistence of porcine coronaviruses in feed and feed ingredients. Almeida A, editor. PLoS One [Internet]. 2017 May 24;12(5):e0178094. Available from: https://dx.plos.org/10.1371/journal.pone.0178094
19. Dee S, Clement T, Schelkopf A, Nerem J, Knudsen D, Christopher-Hennings J, et al. An evaluation of contaminated complete feed as a vehicle for porcine epidemic diarrhea virus infection of naïve pigs following consumption via natural feeding behavior: proof of concept. BMC Vet Res [Internet]. 2014 Aug 5;10:176. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25091641
20. Dee S, Shah A, Jones C, Singrey A, Hanson D, Edler R, et al. Evidence of viral survival in representative volumes of feed and feed ingredients during long‐distance commercial transport across the continental United States. Transbound Emerg Dis [Internet]. 2022 Jan 24;69(1):149–56. Available from: https://onlinelibrary.wiley.com/doi/10.1111/tbed.14057
21. Galvis JA, Corzo CA, Machado G. Modelling and assessing additional transmission routes for porcine reproductive and respiratory syndrome virus: Vehicle movements and feed ingredients. Transbound Emerg Dis [Internet]. 2022 Sep 3;69(5). Available from: https://onlinelibrary.wiley.com/doi/10.1111/tbed.14488
22. Cochrane RA, Schumacher LL, Dritz SS, Woodworth JC, Huss AR, Stark CR, et al. Effect of pelleting on survival of porcine epidemic diarrhea virus–contaminated feed. J Anim Sci [Internet]. 2017 Mar 1;95(3):1170–8. Available from: https://academic.oup.com/jas/article/95/3/1170/4703749
23. Ochoa L, Greiner L, Pacion T. Feed As a Vehicle for Prrs Virus Transmission and the Effects of Formaldehyde on Porcine Reproductive and Respiratory Virus in Feed: Proof of Concept. J Anim Sci [Internet]. 2018 Apr 10;96(suppl_2):63–63. Available from: https://academic.oup.com/jas/article/96/suppl_2/63/4967142
24. Arruda AG, Vilalta C, Perez A, Morrison R. Land altitude, slope, and coverage as risk factors for Porcine Reproductive and Respiratory Syndrome (PRRS) outbreaks in the United States. Raghavan RK, editor. PLoS One [Internet]. 2017 Apr 17;12(4):e0172638. Available from: https://dx.plos.org/10.1371/journal.pone.0172638
You May Also Like



