What’s the future in transdermal devices in swine?
Transdermal devices have many advantages in delivering vaccines and drugs in swine. However, they require excellent training of personnel and excellent maintenance of the equipment.
June 20, 2017

By Christopher Chase, South Dakota State University Department of Veterinary and Biomedical Sciences
Improvements in vaccines and their delivery systems that increase vaccine efficacy, safety or compliance, and minimize animal stress are essential in the swine industry. This column updates our 2008 review in Swine Health And Production1, that reviewed needle-free technology and its uses in disease control in swine.
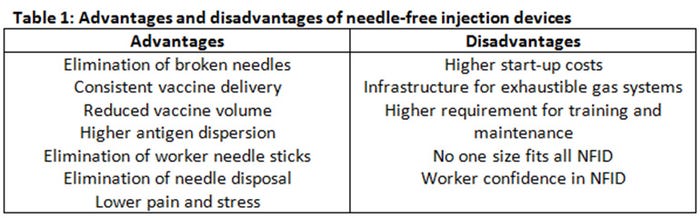
Needle-syringe devices have been the predominant method for vaccine and drug delivery for swine. Although needle-syringe devices are inexpensive and easily adaptable to different settings, needle-free technology offers advantages compared to conventional vaccine delivery methods including elimination of broken needles, consistent vaccine delivery, reduced vaccine volume and higher antigen dispersion, elimination of accidental worker needle sticks, elimination of needle disposal and less pain and stress (Table 1).
The elimination of broken needles and accidental needle sticks are important in the U.S. Pork Quality Assurance program and were targeted by the U.S. National Pork Producers Council in the “One Is Too Many” needle awareness campaign and is part of Good Production Practice 4 “Properly Store and Administer Animal Health Product” of National Pork Board’s PQA Plus Program and the avoidance of accidental needle sticks is part of GPP 8 “Maintain Proper Workplace Safety”. (PQA Plus is now a program of the National Pork Board.)
Needle-free injection devices result in a high-pressure stream that penetrates the epidermis, dermis with some subcutaneous penetration (Figure 1, panel A). NFID-administered swine vaccines can use half to a tenth of the dose required for intramuscular vaccines because of the higher antigen dispersion and contact with the antigen presenting cells found in skin (Figure 2). The number of needle-stick injuries associated with swine workers is unknown, but needle-stick injuries caused the highest number of physical injuries in swine veterinarians with 580 out of 794 (73%) surveyed veterinarians suffering injuries. Thirty-six percent of these injuries resulted in adverse effects (pain, local swelling, hematoma, infection, superficial abscess and cellulitis). In people, the newer generation needle-free devices have been shown to decrease pain and stress at the time of vaccination compared to needle-syringe devices, although there have been some complaints of post-vaccination pain.
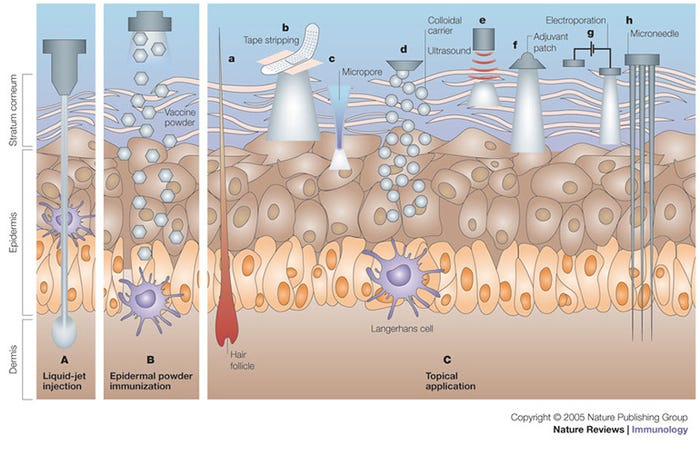
Figure 1: Immunization by cutaneous routes. A) Liquid-jet injection delivers vaccine to muscular, subcutaneous or dermal regions, depending on the parameters of the injection. B) Epidermal powder immunization delivers vaccine powders to the superficial layers of the skin (that is, the epidermis and the superficial layers of the dermis), where they are recognized by Langerhans cells. C)Topical application of vaccines delivers vaccines to the epidermis, where they are recognized and processed by Langerhans cells. Immunization by topical vaccine application is facilitated by several methods. Ca) DNA immunization can be carried out through hair follicles. Cb) Tape stripping removes the stratum corneum and facilitates vaccine absorption. Cc) Thermal or radio-wave-mediated ablation of the stratum corneum creates micropores that increase vaccine delivery. Cd) Colloidal carriers such as microemulsions and transfersomes increase dermal absorption of topically applied vaccines. Ce) Low-frequency ultrasound is an adjuvant for topically applied vaccines, and it also increases vaccine delivery to the skin. Cf) Topically applied adjuvants, such as cholera toxin, can induce potent immune responses. Cg) Electroporation of the stratum corneum increases the delivery of DNA vaccines to the epidermis. Ch) Shallow microneedles that penetrate into the epidermis deliver vaccines effectively. Reprinted with permission from Macmillan Publishers Ltd.5
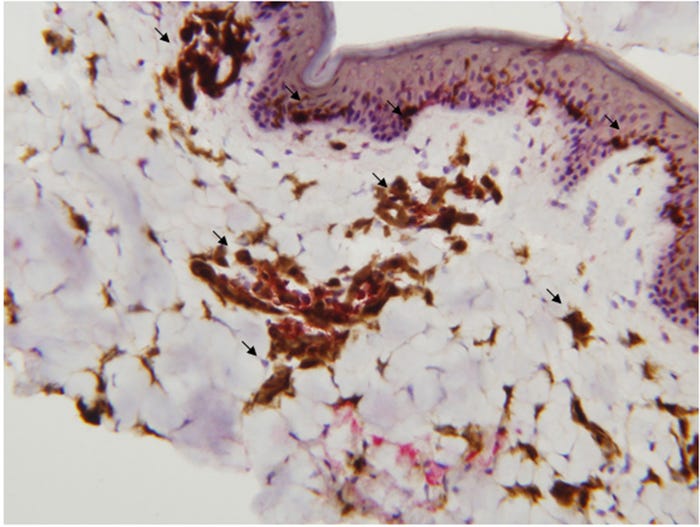
Figure 2: The distribution of dendritic cells in the epidermis, dermis and subcutaneous tissue in the pig. Dendritic cells are stained brown.
Needle-free devices also have disadvantages, including start-up cost of the equipment, exhaustible gas-storage infrastructure (for those systems using compressed or CO2 gas system), technical and operational expertise (training of the operators and maintenance of the units), and inability to completely replace needle-syringe devices in the swine production unit (Table 1). The cost of the equipment varies depending on the type of infector for the needle-free swine units and there are additional associated costs with maintenance and infrastructure especially with compressed gas devices. Needle-free application requires a consistent application method. Needle-free devices are calibrated to deliver the vaccine when the needle-free device is perpendicular (90 degrees) to the skin. Vaccinations made at more acute or oblique angles will affect the distribution of the vaccine in the tissue. In addition, because of the moving parts and gas system, regular maintenance is required.
Finally, there is no “one-size-fits-all” needle-free device for all applications that require injections. Varying pig age, treatment dose and viscosity of injection substance require different injection volume, injection pressure and even different NFIDs (Table 1). Adoption of needle-free devices has increased in the U.S. swine industry since 2008. One major swine processor that processes 5% of pigs in the United States requires that animals never receive needle injections (CS Daniels, personal communication, 2016).
Still the adoption in the United States has been slow. Reasons for this low industry implementation rate involve cost of the unit and associated maintenance and infrastructure costs, higher complexity than needle-syringe device, availability of devices (a smaller handheld injector that is used in Europe is not available in the United States), uncertainty if the animal was vaccinated (i.e., no physical sensation that the animal was vaccinated and/or a “wet” appearance at the injection site), and requirement for training.
There have been several developments with transdermal devices and porcine respiratory and reproductive virus vaccines since the 2008 review. A PRRSV vaccine has been licensed in Europe for use with transdermal devices. Two papers comparing intramuscular needle administration to transdermal administration of PRRSV demonstrated similar immune response vaccines.2,3 More importantly, protection was seen against a heterologous PRRSV challenge at a similar rate between the two routes with one-tenth the volume of vaccine being administered by the TD route.3
One of the reasons for the use of TD devices has been the assumption that these devices would limit animal-to-animal spread compared to needle administration. Vaccination of a PRRSV-positive animal using a TD device spread PRRSV to negative animals 25% of the time compared to 100% in animals with the needle administration.4 Although this certainly decreases spread, it illustrates that PRRSV can be spread by TD devices.
In conclusion, TD devices have many advantages in delivering vaccines and drugs in swine. However, they require excellent training of personnel and excellent maintenance of the equipment.
References
1) Chase, C., Daniels, C. S., Garcia, R., Milward, F., and Nation, T. (2008). Needle-free injection technology in swine: Progress toward vaccine efficacy and pork quality. J Swine Health and Production, 16(5), 254–261.
2) Martelli, P., Cordioli, P., Alborali, L. G., Gozio, S., De Angelis, E., Ferrari, L., et al. (2007). Protection and immune response in pigs intradermally vaccinated against porcine reproductive and respiratory syndrome (PRRS) and subsequently exposed to a heterologous European (Italian cluster) field strain. Vaccine, 25(17), 3400–3408. http://doi.org/10.1016/j.vaccine.2006.12.050
3) Martelli, P., Gozio, S., Ferrari, L., Rosina, S., De Angelis, E., Quintavalla, C., et al. (2009). Efficacy of a modified live porcine reproductive and respiratory syndrome virus vaccine in pigs naturally exposed to a heterologous European (Italian cluster) field strain: Clinical protection and cell-mediated immunity. Vaccine, 27(28), 3788–3799. http://doi.org/10.1016/j.vaccine.2009.03.028
4) Baker, S. R., Mondaca, E., Polson, D., & Dee, S. A. (2012). Evaluation of a needle-free injection device to prevent hematogenous transmission of porcine reproductive and respiratory syndrome virus. Journal of Swine Health and Production, 20(3), 123–128.
5) Mitragotri, S. (2005). Immunization without needles. Nature Reviews Immunology, 5(12), 905–916. http://doi.org/10.1038/nri1728
You May Also Like



