What can you do to help improve ASF surveillance?
Producers and veterinarians have opportunity to help improve ASF surveillance.
October 12, 2021

African swine fever (ASF) has been spreading quickly in the past ten years in several parts of the world, including Asia, Eastern Europe, and more recently in the Dominican Republic and Haiti. This disease causes high mortality in affected pigs, and vaccines do not effectively protect against it. For all these reasons, it is considered one of the main threats to U.S. swine production.
The USDA’s ASF surveillance plan involves testing numerous samples for ASF at the Foreign Animal Disease Diagnostics laboratory, or at one of the partner laboratories of the National Animal Health Laboratory Network (NAHLN). This surveillance plan includes a passive surveillance component and an active surveillance component; the University of Minnesota Veterinary Diagnostic Laboratory (UMVDL) participates in both of these. The passive surveillance component relies on producers and veterinarians identifying cases with clinical signs consistent with ASF, which are then investigated by USDA and state veterinarians through foreign animal disease investigations. The UMVDL is one of 48 NAHLN laboratories authorized to test for ASF in these instances, however such an investigation has only been done once at the UMVDL in the past two years. Therefore, there is room for improvement in this area, and the role of veterinarians and producers is critical to identify candidate cases.
The active surveillance component has three different sources of samples: 1) sick pigs submitted to diagnostic laboratories, 2) pigs from slaughter plants, and 3) pigs from higher-risk farms. The UMVDL is one of 10 NAHLN laboratories involved in testing sick pigs from clinical cases and pigs from slaughter plants. During the period from January 1, 2020 to September 30, 2021, the UMVDL tested 2,900 samples for ASF (Fig. 1).
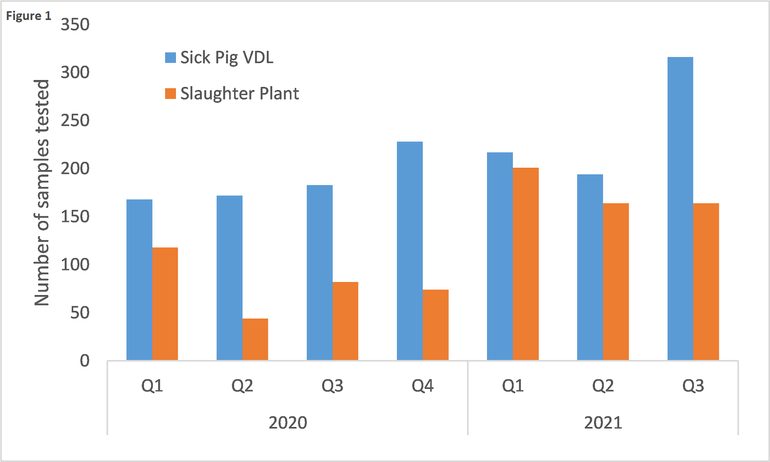
Both sample sources are seeing an increase of tests in 2021 compared to 2020, specifically for sick pig submissions. While the increasing trend is encouraging, there may be room for improvement. During the first nine months of 2021, only 50% of the porcine tissue submissions processed at the UMVDL were included in the surveillance program and tested for ASF. There have been multiple reasons for this. Tissue submissions need to fulfill three conditions to qualify for the surveillance program: a) report clinical signs or lesions that could be compatible with ASF, b) report farm location information (premises ID or address), and c) provide at least one of the ASF approved specimens (tonsil, spleen or lymph node). Unfortunately, very often sample submissions that would otherwise qualify, are not being tested for ASF because they lack the right information or the required samples. For example, if a veterinarian submits lungs and heart form pigs from a case of high mortality, the submission will not qualify because it does not provide any of the approved samples. Similarly, a case of 20% mortality and diarrhea in nursery pigs will not qualify for USDA surveillance if the submitting producer only reports diarrhea as a clinical sign and fails to indicate the increased mortality. Therefore, veterinarians and producers can play an important role in improving the USDA ASF surveillance program of sick pigs.
Now, more than ever, ASF poses a threat to the swine industry. There is a great opportunity for producers and veterinarians to help improve ASF surveillance. First, they can identify and report to USDA and state veterinarians all suspect cases of hemorrhagic disease or marked increase in mortality. Second, then can submit routine diagnostic cases to the laboratory with the appropriate information (clinical signs/lesions and farm location) and approved samples.
More information can be found at the following links:
Sources: Albert Rovira, University of Minnesota, who are solely responsible for the information provided, and wholly own the information. Informa Business Media and all its subsidiaries are not responsible for any of the content contained in this information asset.
You May Also Like



