Is feces still sample of choice for ‘Brachyspira hampsonii’ diagnostics?
For Brachyspira detection, the diagnostic sample of choice is feces, but collection and processing of feces samples create challenges.
January 31, 2017

By Matheus de O. Costa, University of Saskatchewan Western College of Veterinary Medicine and Utrecht University Faculty of Veterinary Medicine; and Janet E. Hill, Champika Fernando, Hollie D. Lemieux and John C. S. Harding, University of Saskatchewan Western College of Veterinary Medicine
Diarrhea, of any kind, is a production limiting condition that directly affects the pig’s ability to absorb nutrients. In the last decade, western Canada and Midwestern U.S. witnessed the re-emergence of bloody, mucoid diarrhea associated with Brachyspira hyodysenteriae (swine dysentery), and now the novel “B. hampsonii” (Rubin et al. 2013; Schwartz 2011; Harding et al. 2010).
Contrary to most intestinal pathogens that affect swine, Brachyspira associated with bloody diarrhea does not attach nor invade the intestinal lining, which may explain why it induces a transient immune response (Rees et al. 1989). This lack of host-bacteria intimate interaction may contribute to the fact that, to date, there is no commercial vaccine available to prevent any Brachyspira spp. infection in domestic animals. Therefore, proper testing of new animals before introduction into a naïve herd is key to avoid outbreaks.
For Brachyspira detection, the diagnostic sample of choice is feces collected freely or after digital stimulation into a clean container (e.g. Ziploc bag), followed by culture on selective agar. Samples need to be refrigerated (not frozen) if delivery to the diagnostic lab will take longer than a couple of hours, which is almost always the case. Because these requirements are challenging to meet when sampling a large number of animals or when farms are distant from diagnostic labs, samples are typically shipped overnight. Sometimes, a more practical alternative to collecting fecal samples is the use of rectal swabs.
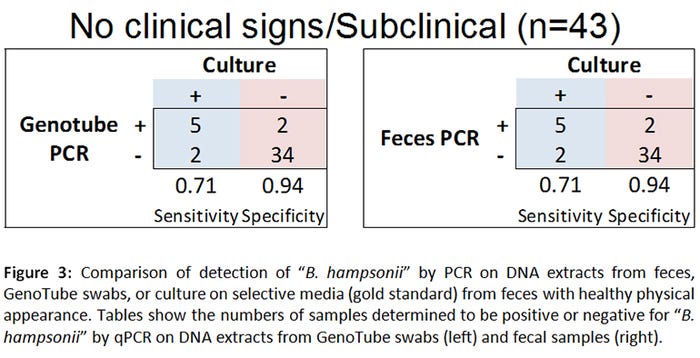
Here we investigated the use of a forensic swab (GenoTube Livestock, Prionics), adapted to preserve DNA at room temperature for extended periods, as an alternative diagnostic tool to test fecal samples for “Brachyspira hampsonii” by polymerase chain reaction. We compared the detection frequency of “Brachyspira hampsonii” PCR performed on rectal GenoTube swabs, to PCR performed directly on a sample of feces, to anaerobic culture of a standard rectal swab on Brachyspira-specific agar (gold standard). To simulate a field situation where samples would be transported to the diagnostic laboratory over 24 hours, culture swabs and feces were refrigerated at 4 degrees C (to mimic shipment with ice packs), whereas GenoTube swabs were stored at room temperature for 24 hours prior to processing. To ensure consistent sample expression, all swabs were processed the exact same way. Following expression, extraction proceeded according to the kit instructions. DNA extracts (2 microliters each) from standard culture swabs and GenoTube swabs were used as a template for “B. hampsonii” clade I specific PCR analysis.
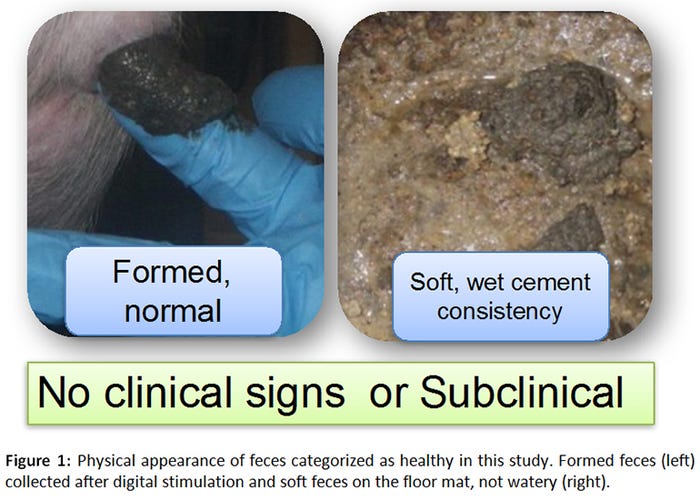
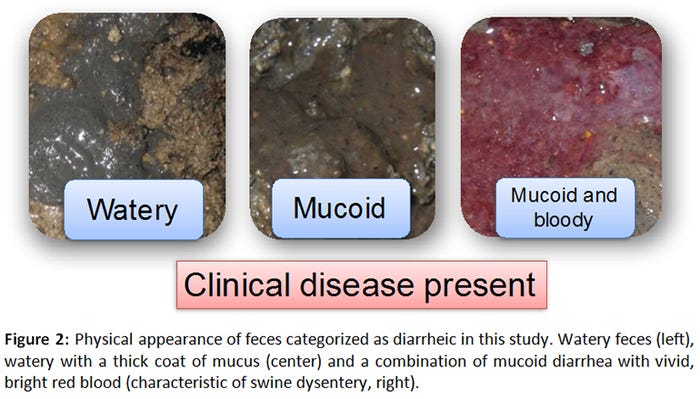
A total of 165 samples (55 GenoTube swabs, 55 culture swabs, 55 fecal samples) were collected from 12 orally inoculated animals over a period of 22 days. Seventy-eight percent of samples (43/55) were obtained from pigs with normal feces (Figure 1), whereas 22% (12/55) originated from pigs with varying severities of diarrhea (Figure 2).
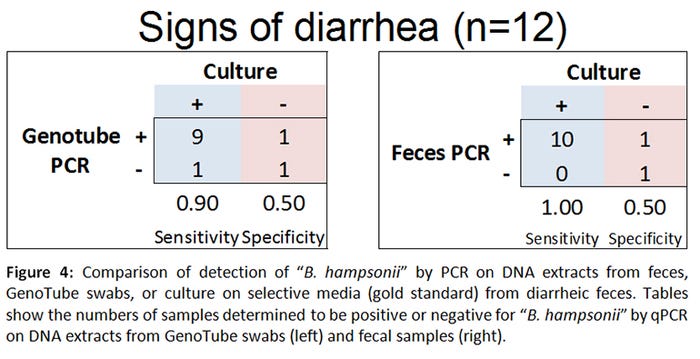
When compared to the gold standard (rectal swab culture on selective agar), PCR from GenoTube swabs from animals with no clinical signs of diarrhea performed identically to PCR from fecal samples (Figure 3). In animals with diarrhea, PCR performed directly on fecal samples was superior to PCR performed on GenoTube swabs (Figure 4). The odds of a positive test result were higher when PCR performed directly on feces compared to on a GenoTube swab. However, it is important to note that these results are based on a small controlled inoculation study, with most samples collected from animals without diarrhea. Additional analyses using field cases would be beneficial.
GenoTube may be a useful tool for diagnostic and surveillance projects, especially in settings where timely transport of diagnostic samples is challenging. The collection of rectal swabs is easy and efficient when sampling large numbers of pigs, resulting in minimal stress to the pigs and collectors. However, a combination of factors including the amount of sample available in the swab and laboratory processing procedures may have influence in the performance of the PCR assay, decreasing sensitivity (Bonini et al. 2002; Plebani and Carraro 1997). Cases where sample collection and shipping conditions are not an impediment, fecal samples are still the diagnostic sample of choice.
References
Bonini, P., Plebani, M., Ceriotti, F., & Rubboli, F. (2002). Errors in laboratory medicine. [Review]. Clinical Chemistry, 48(5), 691-698.
Harding, J. C. S., Chirino-Trejo, M., Fernando, C., Jacobson, M., Forster, Z., & Hill, J. E. Detection of a novel Brachyspira species associated with haemorrhagic and necrotizing colitis. In International Pig Veterinary Society Congress, Vancouver, Canada, July 18-21, 2010 2010 (Vol. 2, pp. 740)
Plebani, M., & Carraro, P. (1997). Mistakes in a stat laboratory: types and frequency. Clinical Chemistry, 43(8 Pt 1), 1348-1351.
Rees, A. S., Lysons, R. J., Stokes, C. R., & Bourne, F. J. (1989). Antibody production by the pig colon during infection with Treponema hyodysenteriae. Research in Veterinary Science, 47(2), 263-269.
Rubin, J. E., Costa, M. O., Hill, J. E., Kittrell, H. E., Fernando, C., Huang, Y., et al. (2013). Reproduction of mucohaemorrhagic diarrhea and colitis indistinguishable from Swine Dysentery following experimental inoculation with “Brachyspira hampsonii" strain 30446. PLoS ONE, 8(2), e57146, doi:10.1371/journal.pone.0057146.
Schwartz, K. (2011). Brachyspira: What's Happening in Iowa and Why. Paper presented at the Western Canadian Association of Swine Veterinarians Conference, October 20-21, 2011
You May Also Like



