Here’s a genome editing and CRISPR-Cas9 primer
Although these technologies seem like science fiction, small changes to the DNA sequence of an organism are common within individual cells in the body and after fertilization of an oocyte by sperm.
April 2, 2019

By Blythe Schultz and Jason W. Ross, Iowa State University Department of Animal Science Iowa Pork Industry Center
Genome editing (also known as gene editing, genome engineering, genetic engineering) refers to a set of tools and techniques that allows researchers to manipulate the underlying genetic code of an organism. These changes can include adding, removing or making specific modifications to targeted regions in the genome. Although a number of methods exist for genome editing, the potential promise and quick rise to popularity of the CRISPR-Cas9 approach, which stands for Clustered Regularly Interspaced Short Palindromic Repeats and CRISPR associated protein 9, has placed it front and center in the public eye.
CRISPR-Cas9 was originally discovered in bacteria where it protects against viruses in a similar way to how our immune system operates. By storing short pieces of DNA from the viruses, the bacteria are able to recognize viruses that have infected them before and produce short RNA sequences that match the viruses’ DNA. These short RNA sequences bind to Cas9, or other CRISPR associated proteins, which are able to cut both strands of the targeted DNA. If the virus is unable to correctly repair these cuts, it usually dies. Much like in the bacteria, researchers are able to create short RNA sequences that bind to Cas9 and guide it to specific locations of interest within an organism’s genetic code. Once a cut is made, researchers rely on the cell’s own DNA repair mechanisms to make the desired manipulations (add, remove or modify).
Although these technologies seem like science fiction, small changes to the DNA sequence of an organism are common within individual cells in the body and after fertilization of an oocyte by sperm. The ability to tightly control these changes is very exciting for researchers in a number of different fields. Currently, some of the biggest areas of interest include using these technologies to treat human disease, to create animal models of common genetic diseases in humans, and to improve the welfare and performance of livestock species. The use of these technologies to improve livestock is sometimes referred to as “precision breeding,” because many of the genetic changes that are made also occur naturally and could be bred into production livestock over many generations. By using precision breeding, the same improvements can be seen in fewer generations.
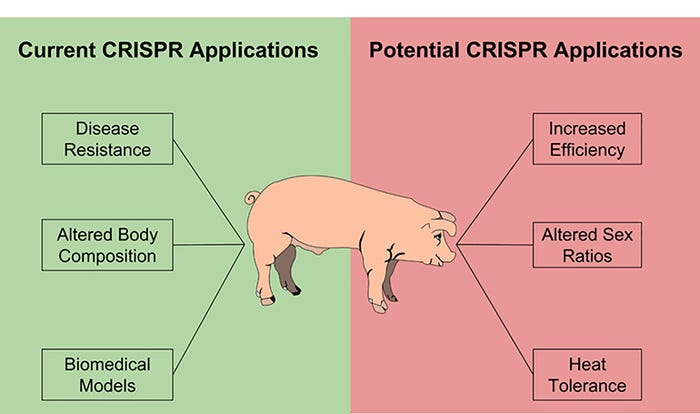
Current opportunities for the use of genome editing in livestock includes introducing beneficial traits that are controlled by one gene, removing genes that increase disease susceptibility and other production losses, and manipulating many genes that make small contributions toward increased production. Due to the limited number of traits that are controlled entirely by a single gene and the increased difficulty of introducing desired traits, there are fewer examples of this type of modification to date. One agriculturally relevant example of genetic modification is the introduction of UCP1, a protein that pigs lack, which resulted in pigs that were more cold tolerant and had 5% less fat at slaughter. The insertion of specific sequences is particularly helpful in studying human diseases, because a number of diseases are the result of changes to one gene. Pigs serve as a great biomedical model, and CRISPR/Cas9 has allowed researchers to develop pigs that reliably mimic human diseases, such as Huntington’s disease, in order to better understand and treat them.
One of the most common types of manipulation being performed in livestock right now is the removal, or knock out, of genes that play a role in disease susceptibility. Two examples of this from Randy Prather’s group at the University of Missouri are pigs that lack functional CD163 and ANPEP genes. The deletion of these genes results in offspring that are resistant to the porcine reproductive and respiratory syndrome virus and the transmissible gastroenteritis virus, respectively.
Although there are not currently many published examples, the potential to improve pig production by manipulating a number of genes that all contribute a small amount to economically valuable traits is very exciting. The ability to make a large number of mutations in pigs at one time has already been demonstrated in the context of creating pigs for biomedical research, and methods and technologies for identifying the genes that contribute to production traits continue to improve. The convergence of these technologies will allow us to rapidly improve pig production much the same way as genetic selection.
While gene-edited livestock are not currently approved by the Food and Drug Administration for consumption, other agricultural technologies using genetic modification, such as the AquaBounty salmon, have been approved indicating that future opportunities to capitalize on the technology for the benefit of animals, consumers and producers is a realistic possibility. An understanding of how the technology works, its potential and limitations, and collaboration between producers and researchers to meet the most urgent needs of the industry will ensure that this powerful new tool benefits all levels of production, as well as the consumer.
Source: Blythe Schultz and Jason W. Ross, who are solely responsible for the information provided, and wholly owns the information. Informa Business Media and all its subsidiaries are not responsible for any of the content contained in this information asset.
You May Also Like



