Gilt acclimation
Blueprint: Controlling Mycoplasma hyopneumoniae infections even before they start.
October 22, 2019

By Maria Pieters, University of Minnesota; Alyssa Betlach, Swine Vet Center; and Amanda Sponheim, Boehringer Ingelheim Animal Health
Whether a recent outbreak or an endemic infection, a single pathogen issue or a polymicrobial complex, Mycoplasma hyopneumoniae (M. hyopneumoniae, termed mycoplasma for the purpose of this article) is at the heart of respiratory disease in swine.
Producers and practitioners frequently deal with the chronic respiratory condition caused by this pathogen and with the consequences of such infections, which have been recognized for more than half a century and have received significant veterinary attention on herd visits.
In this article, we comment on the use of gilt acclimation as a key strategy for mycoplasma control in swine herds — what works, what doesn’t and where we should go from here.
Work to control mycoplasma
There are three main ways in which mycoplasma control is commonly attempted: the modification and optimization of management practices, application of vaccine products and use of antimicrobial agents. Segregated production within farm pig density, pig flow and barn ventilation, among others, are management practices that can be modified to maximize respiratory health and avoid disease transmission and dissemination.
Bacterins are regularly included in farm vaccination programs in order to reduce clinical signs of disease and minimize the effect of the disease on production parameters. In addition, antimicrobials are administered to infected pigs to decrease bacterial burden and the accompanying clinical signs.
Altogether, the selection and use of control methods (or most commonly, their combination) is based on the specific clinical presentation and production system’s characteristics. However, no matter what approach is taken, most would agree that mycoplasma control is partial, unless disease elimination is pursued.
Long-term perspective change
Historically, the industry’s perspective on mycoplasma control has been primarily focused on managing the infection at the farm or herd level — especially as it pertains to finishing herds, where clinical signs of the disease are often evident.
While this approach is necessary, the ability to control mycoplasma in the long term is difficult to achieve in this manner. With a more comprehensive understanding of mycoplasma epidemiology and health instability at sow farms, incoming gilts have been recognized as key factors for the circulation and perpetuation of this pathogen within flows.
Several studies have shown that the primary source of mycoplasma infection originates from shedding dams during farrowing and lactation. Piglets are often colonized early in life, and due to the slow and chronic nature of the infection, clinical signs aren’t often evident until later.
Therefore, it is important to focus our efforts and implement control strategies before the start of infection (i.e., at the sow farm).
One important aspect of disease control is that each herd is a unique production unit, with specific characteristics that will largely determine the potential methods and strategies to be applied. Thus, knowing the mycoplasma status of the sow farm as well as of the incoming gilts is the first step toward designing a plan of work.
For example, in mycoplasma-negative sow farms, regular gilt entry — necessary for production and genetic improvement purposes — is a constant risk for disease introduction. Thus, strict diagnostic evaluation and confirmation of freedom of infection in gilts is of paramount importance. A small proportion of unknowingly infected gilts allowed into a negative sow farm would end up in a clinical outbreak, with significant welfare, personnel morale and economic implications.
On the other hand, mycoplasma-positive sow farms may operate in an endemic (desired to be stable) state, or embark on disease elimination programs. Regardless of the decision to live with or to get rid of mycoplasma infections, gilt acclimation remains central to achieving long-term disease control.
Gilt acclimation has evolved
Various strategies are used for controlled exposure of gilts to mycoplasma prior to entering the sow farm. Over the years, health professionals have attempted to avoid issues in herds endemically infected by mycoplasma by applying vaccination and medication in an effort to make sure that gilts perform well in recipient farms, which for the most part worked for disease control.
Nevertheless, one factor that has changed for most producers is the fact that replacement gilts are now supplied negative (naïve) to mycoplasma. While this is desirable for mycoplasma-negative herds, it poses challenges when incorporating the susceptible gilts into farms in which mycoplasma disease is endemic.
The result of this situation (based on the age of gilt introduction) can be difficult to deal with from various standpoints. First, susceptible gilts will eventually be infected with the pathogen and will develop disease. This can happen soon after entry; but since mycoplasma is a pathogen that is transmitted slowly among pigs, this process can take weeks, even months to occur.
Thus, constant infections are occurring in the herd, which maintains circulation of the pathogen to new susceptible hosts — constituting the basis of a never-ending mycoplasma story in sow farms.
To further complicate matters, newly infected gilts or sows may shed the bacterium for long periods, which have been shown to reach as long as seven months after initial infection in research experiments. This later statement implies that dams may go through the production cycle and eventually farrow while shedding mycoplasma, potentially colonizing baby pigs.
Piglet colonization will spread the pathogen and accompanying disease to the nursery and finishing phases, all the way to market. For those reasons, the need to perform early and purposeful acclimation of gilts for mycoplasma has been highly recognized in recent years.
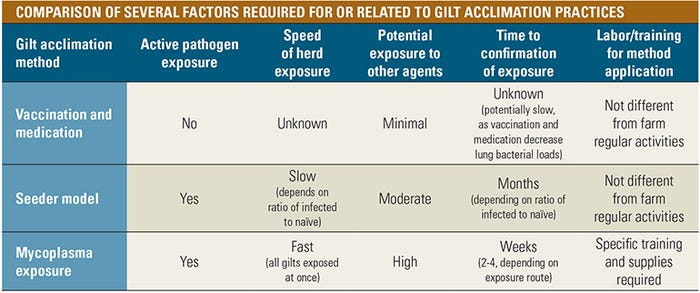
Gilt acclimation methods
There are several methods for gilt acclimation:
Vaccination and/or medication. Considered the most conservative approach for gilt acclimation, the combined use of vaccine and antimicrobials for disease control in gilts had been generalized in the industry.
This type of approach is especially common in farms that are expected to be negative and use bacterins as a shield for unexpected infections. However, the limitations inherent to these tools for gilt acclimation, such as the incomplete protection provided by bacterins and the inability of medications to eliminate mycoplasma from the gilt, have driven many to the use of more involved options.
Mycoplasma seeder model. Given the fact that infections with mycoplasma are usually maintained in herds, it seems logical to think that older gilts and sows can act as “seeders,” amplifying and shedding the bacterium to younger gilts. In fact, this type of mycoplasma exposure has been widely employed for gilt acclimation purposes. However, the nature of this pathogen is the limiting factor for this type of exposure.
Several experiments and field-based studies have shown that transmission of mycoplasma among pen mates is a slow event that requires several weeks to be completed in groups. It has been proposed that the same number of infected gilts should be in close contact with susceptible gilts, if the expectation is for all gilts to be infected in a relatively short period of time (four weeks).
In reality, this is a very difficult scenario to apply from the practical point of view, as space and facility requirements would double, housing a significant proportion of nonselected or culled gilts and sows just to spread the pathogen. Even if a 1-1 ratio of seeder to susceptible gilts can be achieved, the exposure process would need to begin before 50 days of age to allow for gilts to recover from disease and not shed mycoplasma to their piglets at first farrowing.
Furthermore, the confirmation of determining nonselected or culled gilts as seeders can be difficult at times. To confirm, diagnostics should be used to aid in identifying proper seeders and monitoring exposure, which is a critical component to the success of the model.
Mycoplasma exposure. New methods for gilt exposure have evolved to minimize transmission time, facility space and seeder gilt or sow requirements for a seeder model. This has been achieved by administering a herd-specific lung homogenate to individual gilts or to large groups of gilts at once.
While mycoplasma exposure to large groups may decrease transmission time by approximately 30 to 50 days by generating a uniform population exposure when compared to an ideal seeder model, extensive diagnostic pre-work, procedural training, specific supplies and involvement of a veterinary professional are necessary.
To select the appropriate donor gilt for the lung homogenate, diagnostic pre-work is required to confirm mycoplasma infection and to screen for other respiratory pathogens. Furthermore, depending on the volume of lung homogenate needed for the exposure method and the initial availability of donor pigs, another four weeks may be needed to generate additional donors.
Individual exposure is labor-intensive and involves proper pig restraint and administration of the homogenate by trained professionals using specialized equipment. In an attempt to find an approach for exposure that minimized handling stress to gilts and personnel labor demands, field swine professionals developed a method of large group exposure that involved inhalation of the material containing the bacterium.
For both exposure methods, and similar to a seeder model, diagnostics should be applied to confirm population exposure has occurred. Exposure confirmation has been achieved as early as two weeks following large-group exposure.
Furthermore, vaccination and medication strategies should be judiciously applied to lessen the severity of disease following exposure, if needed, and a veterinary professional should evaluate necessary adjustments to the exposure method if commonly measured production parameters are negatively impacted.
Attempts to eliminate disease
One interesting aspect of gilt acclimation practices is that even though they may be mainly directed at generating a uniform exposure of the herd to mycoplasma for stabilization purposes, they also represent a good strategy to establish the beginning of Day 0 for disease elimination.
Day 0 represents the first step toward counting the number of days a herd will be closed to gilt introductions, and it constitutes the basis of a successful elimination program. mycoplasma exposure has become a frequently employed method for establishing Day 0. Due to the importance of the accuracy of this time point in an elimination program, it is essential that appropriate diagnostics be used to confirm the gilt population has been effectively and uniformly exposed.
In summary, gilt acclimation for mycoplasma in sow farms is an area of intense research and activity nowadays, especially based on the significant influence it can have in all phases of swine production. Information on pathogen transmission and disease dynamics has guided the development of tools and methods to tackle herd instability issues.
However, current strategies for gilt acclimation may benefit from further refinement, and can and should be improved to confer more uniformity, predictability and safety. Efforts for improved gilt acclimation need to continue in order to offer solutions to swine producers and professionals, and to measurably increase overall pig health and welfare in sow farm and downstream herds.
Pieters is an assistant professor with the Department of Veterinary Population Medicine and Veterinary Diagnostic Laboratory at the University of Minnesota. Betlach is an associate veterinarian with the Swine Vet Center. Sponheim is a technical services veterinarian with Boehringer Ingelheim Animal Health USA Inc.
Sources: Maria Pieters, Alyssa Betlach and Amanda Sponheim, which is solely responsible for the information provided, and wholly owns the information. Informa Business Media and all its subsidiaries are not responsible for any of the content contained in this information asset.
You May Also Like



