Gender and stress matter in pig gut health
Blueprint Issue: An overview of gut health and highlight recent research demonstrating how wean age and sex (i.e., being female or male) influence gut development and health throughout the lifetime of the pig.
April 17, 2018

By Adam Moeser, M.S., DVM, Ph.D., Matilda R. Wilson Endowed Chair, Michigan State University Department of Large Animal Clinical Sciences’ Gastrointestinal Stress Biology Laboratory
As the U.S. swine industry transitions away from antimicrobial growth promotants, an increased focus has been placed on developing alternative methods to preserve gut health and animal performance. However, the underlying biological basis of many gut health disorders in pigs remains poorly understood, and thus well-defined and predictable biomarkers and biological targets for modifying gut health are lacking.
This represents a large gap in knowledge, with profound health, welfare and economic implications to the U.S. swine industry.
Therefore, there remains a critical need for fundamental research investigating important factors (e.g., environmental and biological) that impact gut development and disease risk in pigs, which will be essential for the discovery of targeted, effective gut health therapeutics and the identification of potentially new management practices. This article will provide an overview of gut health and highlight recent research demonstrating how wean age and sex (i.e., being female or male) influence gut development and health throughout the lifetime of the pig.
What is gut health, and how is it measured?
This topic has been subject to much debate as we continue to search for true biomarkers that reflect gut health. Gut health is best defined in terms relative to the critical functions the gut performs on a daily basis. First, the gut must provide a selective permeability barrier (aka barrier function) to limit or tightly regulate the exposure of environmental antigens (e.g., feed antigens), toxins and microorganisms to the gut mucosal immune system. Excessive intestinal permeability causes intestinal inflammation, and in severe cases, sepsis and death. Second, the gut is an important immune barrier that includes the ability to initiate a rapid and robust immune response required for clearance of pathogens. The gut immune system also functions to establish an immunologically tolerant environment to educate and develop and prevent overwhelming inflammatory responses in the highly antigenic gut lumen. Third, the gut must facilitate nutrient uptake (e.g., nutrient digestion, absorption and metabolism) to support overall organ system function and growth (Figure 1).
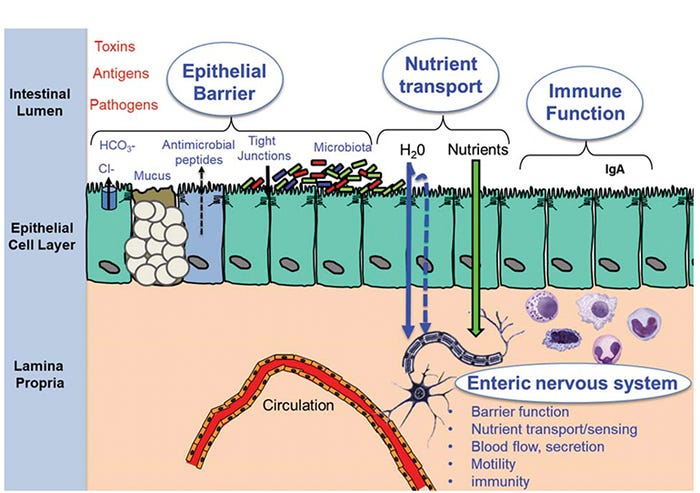
Figure 1: Major components of gut health
While seemingly distinct, there is considerable overlap in gut functions. For example, in addition to optimal growth, efficient nutrient absorption is required during the time of stress and disease challenges to support critical functions of the immune system.
Growth performance and feed efficiency measurements have been classically used as indexes of gut health. However, this measure alone does not always reflect optimal gut health.
Under specific environmental conditions such as high health status or concurrent use of antibiotics, pigs can exhibit good performance and be disease-free despite subtle defects in underlying gut dysfunction (e.g., increased gut permeability or leaky gut, poorly developed or suppressed gut immune function, etc.). The impact of gut dysfunction on performance and disease may not be revealed unless the pig is faced with production stressors or an infectious challenge, both common in swine production.
Stress impacts gut health
In swine production, pigs are exposed to numerous stressors including weaning, temperature fluctuations (cold and heat stress), mixing and transport — which is known to erode gut health and performance and increase the vulnerability to infectious pathogens. The ability of the pigs to respond and resolve the stress, i.e., their stress resilience, is a major determinant of whether they will exhibit compromised gut health and disease resistance.
On the one hand, the pig must be able to initiate a robust physiologic response to stress which involves activation of the fight-or-flight response — a conserved mechanism critical for survival. The stress response is mediated largely by activation of the hypothalamic pituitary adrenal axis (aka fight-or-flight response), which is critical for mobilization of body resources (e.g. blood flow, nutrients), enhancement of immune function, and defense and initiation of behaviors that help to cope with the stress and return to homeostasis once the stressor has been dealt with or adapted to.
Alternatively, the stress response can often become detrimental, such as in situations where the stressors are overwhelming (e.g., multiple concurrent stressors or a high stress load) or chronic in nature, which can exhaust coping mechanisms and prevent the return to homeostasis.
The timing of stress matters. Early-life stress induces long-term detrimental effects on gut health.
Weaning is the most stressful period in pig production. During the weaning period, the young pig is exposed to multiple concurrent stressors including maternal separation, separation of littermates and disruption of social hierarchy, abrupt changes in diet and environment, and often long transportation times. Importantly, weaning stress occurs during a major postnatal development window, where many biological systems in the pigs are undergoing extensive development and maturation (Reviewed by Pohl et al., 2016; Moeser et al., 2018).
For example, in the first three to four months of postnatal life, there is extensive development and programming of the HPA axis stress response system, intestinal barrier function, and gut immune and nervous systems.
It is therefore not surprising that the length of postnatal gut development coincides with the natural weaning process in wild pigs, which is gradual and occurs at three to four months of age. Because environmental influences play a key role in shaping postnatal development of the gut (and other systems), significant stress or trauma can alter the programming of a gut function, having potentially lasting detrimental impacts.
Our previous work and that of others demonstrate that early weaning in production induces significant gut barrier injury, characterized by increased intestinal permeability and intestinal inflammation. Further, we demonstrated that nursery-age pigs that were previously early-weaned (<23 days of age) exhibit increased intestinal permeability (compromised barrier function) that persists into adulthood (Pohl et al., 2017) and exhibit more severe disease and performance reductions, compared with later-weaned pigs, in response to a challenge with postweaning F18 E. coli (McLamb et al., 2010).
Original research by Roger Main et al. (2004) and more recently by David Rosero and Dean Boyd (Hanor Farms, Proceedings from the Al Leman Conference, 2016) showed increased disease risk and performance reductions associated with early wean ages in field conditions. Together, basic and applied research support the concept that trauma or stress during in critical developmental windows in early life alters the gut developmental trajectories toward impaired gut function and increases disease risk throughout the life span (Figure 2).
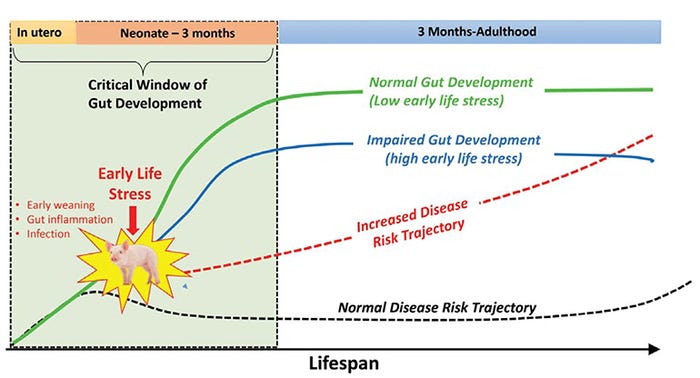
Figure 2: Early life stress changes the trajectory of gut development and increases lifetime disease risk.
Sex and gut health
Biological sex (i.e., being male or female) has long been known as a significant risk factor for many diseases in people. For example, irritable bowel syndrome, a functional bowel disorder that affects up to 25% of the population, is two to four times more common in women compared to men. Similar sex difference trends are found for gastrointestinal parasite-related diseases, while inflammatory bowel disease and GI cancer are often found in higher rates in men.
Do sex differences exist in gut health disorders in pigs? The effects of sex on growth performance and feed efficiency has been extensively studied. It is also well-established that preweaning and postweaning mortality rates (resulting from many causes) are higher in male pigs compared with gilts. Straw et al. (2001) reported in one study that barrows exhibited a higher death loss from hemorrhagic bowel syndrome during summer months. The authors also reported that deaths from other causes were 1.7 times higher in barrows, compared with gilts. While the studies above certainly support that sex plays an important role in disease and mortality in pigs, whether sex plays a role in gut health has not been extensively investigated.
Study objective: To determine how biological sex and early weaning impacts long-term gut development and function in pigs
Study design: Gilts and castrated barrows (Yorkshire crossbred) were randomly divided into early-weaning (16- to 17-day wean age) or late-weaning (28- to 30-day wean age) experimental groups. Weaned pigs were co-housed in the same nursery according to standard guidelines for stocking density. Pigs were fed the same phase feeding program and identical diets formulated to meet or exceed nutrient requirements for the pig (NRC Swine, 2012). Pigs were allowed ad libitum access to feed and water.
At late-nursery (60 days of age) and finisher (170 days of age) stages, gut health and function were assessed by conducting intestinal function assays (intestinal permeability, nervous system and secretory activity) and histological and biochemical assays. Also, fecal scores were performed throughout the study by evaluators, blinded to experimental groups, as a clinical index of gut function. Full details of the experimental procedures are described in Medland et al. (2016) and Pohl et al. (2017).
Results
Early-weaned gilts exhibit more severe and persistent intestinal barrier defects and greater incidence and severity of diarrhea compared with barrows.
Intestinal permeability, a sensitive index of gut barrier injury, was measured, and fecal scores were recorded as a general clinical index of function in nursery and finisher stages. In late-weaned pigs, intestinal permeability and fecal scores were similar between gilts and barrows. However, in early-weaned pigs, significant sex differences were revealed, with gilts exhibiting greater intestinal permeability (by about 1.5-fold) and more time exhibiting loose stools (58.8% vs. 29.9%, p=0.0016), in nursery and finisher stages.
Early-weaned gilts develop a hypersensitive gut nervous system and increased mast cell activity.
The GI tract contains an abundance of neurons, equaling that of the spinal cord. The gut nervous system plays a role in essentially all aspects of gut health including epithelial nutrient transport and barrier function, secretion and absorption, immunity and motility. In pig gut health disorders associated with stress, infectious pathogens (e.g., Salmonella typhimurium, Rotavirus, E. coli, etc.) cause hyperactivation of nerves in the gut, which drives hypersecretion of fluid, and hypermotility, which results in diarrhea.
We, therefore, examined whether wean age and biological sex influenced the development of the gut nervous system. Similar to intestinal permeability and fecal scores, late-weaned gilts and barrows had similar numbers of gut neurons and activity (determined by measuring secretory function in response pharmacologic nerve activation).
However, in early-weaned pigs, gut neurons were higher in number and displayed enhanced sensitivity to nerve-induced secretory response, reflective of a hyperreactive gut nervous system. Further, early-weaned pigs expressed a different profile of neurotransmitters within their gut neurons characterized by increased expression of acetylcholine — the predominant neurotransmitter system regulating fluid secretion, motility and immune function in the gut.
While early-weaned barrows and gilts both exhibited increased numbers of gut neurons in the late nursery and finisher phases, early-weaned gilts had enhanced sensitivity to nerve-induced secretory function compared with early-weaned barrows.
Mast cells are innate immune cells that play critical roles in host defense against environmental allergens and pathogens. In the gut, mast cells are located near blood vessels, neurons and gut epithelial cells. When activated, they rapidly release mediators (e.g., histamine, proteases, serotonin and cytokines) that induce rapid and profound effects on gut blood supply, permeability and immune responses.
Our previous work showed that mast cell activation in pigs is enhanced in response to early weaning, which was shown to be a major pathway causing increased intestinal permeability in early-weaned pigs (Moeser et al., 2007; Smith et al., 2009). Given the central importance of gut mast cells in gut health, we examined whether sex differences exist in mast cell activity, and how this is influenced by early weaning.
These investigations revealed that early-weaned gilts and barrows exhibited higher numbers of intestinal mast cells throughout their GI tracts, compared with late-weaned pigs; however, no sex differences were observed in mast cell numbers between the sexes.
However, when measuring mast cell mediator release, significant sex differences were found, with early-weaned gilts exhibiting greater amounts of mast cell protease release, compared with barrows. This sex effect in mast cells was also shown in other animal models of stress (Mackey et al., 2016). A summary of sex differences is provided in Figure 3.
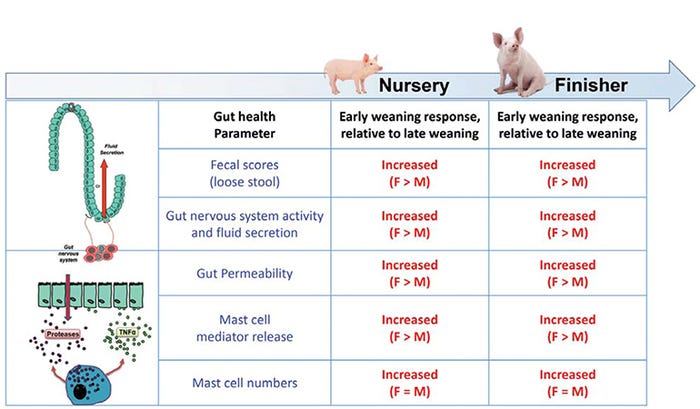
Figure 3: Impact of early-weaning stress and sex on gut health throughout the life span
Conclusions
Here are the take-home messages from the study:
■ Gut health is best defined and measured by assessing the critical functions it performs, including barrier function, nervous and immune function, and nutrient uptake.
■ Early life stressors (e.g., weaning) have long-lasting, deleterious impacts on gut health and disease risk, and they compromise performance throughout the production life span.
■ Biological sex plays a major role in gut health. Gilts exhibit heightened nervous and immune activation, increased intestinal permeability and increased diarrhea. However, the fact that gilts exhibit lower mortality rates than barrows or boars suggests that the heightened gut reactivity in females might confer an advantage over males for survival.
■ More basic fundamental and applied research is needed to determine the mechanisms of sex differences between gilts and barrows in gut health disorders, which would be expected to uncover novel targets and strategies to optimize gut health in both sexes.
References
Main, R.G.; S.S. Dritz; M.D. Tokach; R.D. Goodband; and J.L. Nelssen. “Increasing weaning age improves pig performance in a multisite production system.” J Anim Sci. 2004 May;82(5):1499-507.
Mackey, E.M.; S. Ayyadurai; C.S. Pohl; S. D’Costa; Y. Li; and A.J. Moe-ser. “Sexual dimorphism in the mast cell transcriptome and the pathophysiological responses to immunological and psychological stress.” Biology of Sex Differences 7:60, 2016. PMCID: PMC5120457
McLamb, B.L.; A.J. Gibson; E.L. Overman; C.H. Stahl; and A.J. Moe-ser. “Early Weaning Stress in Pigs Impairs Innate Mucosal Immune Responses to Enterotoxigenic E. coli Challenge and Exacerbates Intestinal Injury and Clinical Disease.” PLoS One. 8:e59838, 2013.
Medland, J.E.; C.S. Pohl; L.L. Edwards; S.F. Frandsen; K. Bagley; Y. Li; and A.J. Moeser. “Early life adversity in piglets induced long-term upregulation of the enteric cholinergic nervous system and heightened sex-specific secretomotor neuron responses.” Neurogastroenterol Motil. 28:1317-29, 2016. PMCID: PMC5002263; NIHMSID: NIHMS767094.
Moeser, A.J.; C.S. Pohl; and M. Rajput. “Weaning stress and gastrointestinal barrier development: Implications for lifelong gut health in pigs.” 2017 Animal Nutrition 3 (4) 313-321. https://doi.org/10.1016/j.aninu.2017.06.003
Pohl, C.S.; J.E. Medland; and A.J. Moeser. Invited Review: “Early Life Stress Origins of Gastrointestinal Disease: Animal Models, Intestinal Pathophysiology, and Translational Implications.” Am J Physiol Gastrointest Liver Physiol. 309(12):G927-41, 2015. PMCID: PMC4683303.
Pohl, C.S.; J.E. Medland; E. Mackey; L.L. Edwards: K.D. Bagley; M.P. Dewilde; K.J. Williams; and A.J. Moeser. “Early weaning stress induces chronic functional diarrhea, intestinal barrier defects, and increased mast cell activity in a porcine model of early life adversity.” 2017 Neurogastroenterology and Motility. doi: 10.1111/nmo.13118, PMID: 28573751.
Rosero, D.S.; T. Donovan; and R.D. Boyd. “Influence of Wean Age and Disease Challenge on Progeny Lifetime Performance.” Presentation at Al Leman Conference, 2016.
Smith, F.; J.E. Clark; E.L. Overman; C.C. Tozel; J.H. Huang; J.F. Rivier; A.T. Blikslager; and A.J. Moeser. “Early weaning impairs postweaning development of intestinal barrier function in porcine jejunum.” Am J Physiol Gastrointest Liver Physiol. 298(3):G352-63, 2010.
You May Also Like


