Adapting the PRRS oral fluid ELISA to routine surveillance
Using a cut-off of S/P≥1.0 for routine PRRSV surveillance is recommended, as data show this cut-off will improve the diagnostic specificity of the test.
November 3, 2020

Routine porcine reproductive and respiratory syndrome virus surveillance requires (1) a population-based specimen that is easy to collect and (2) the availability of accurate diagnostic tests. Oral fluids (OF) are easily collected, represent the population from which they have been collected, and are currently the most common surveillance specimen in U.S. veterinary diagnostic laboratories1. Both PRRSV RNA (real-time polymerase chain reaction) and antibody (enzyme-linked immunosorbent assay) testing have been widely researched and are well-established tests in our laboratories.
When oral fluids are used for routine PRRSV surveillance, test selection (RT-PCR, ELISA or a combination of the two) should depend on the surveillance objectives and specific herd circumstances. In general, detection of PRRSV RNA provides the advantage of early detection, whereas PRRSV antibody provides the advantages of lower cost and longer duration of detection. The coordinated use of both can provide the most complete characterization of the population2.
People often think that extremely sensitive tests are required for surveillance (we must find every positive!). This is true in some cases (boar studs, for example) but not in others. In most production settings, the test must provide adequate detection (diagnostic sensitivity), but perfect diagnostic sensitivity is not necessary because the infection will be discovered at the next sampling (assuming you are surveillance sampling on a regular basis).
In contrast, near-perfect diagnostic specificity is required for surveillance because the cost of "unexpected positives" is high both on the farm and in the lab. "Unexpected positive" results invariably cause disruption in the workflow on the farm and in the lab, raise blood pressure and usually require re-sampling, retesting, or both. If ultimately determined to be a false positive, people quickly lose confidence in both the laboratory and the test. Achieving near-perfect diagnostic specificity usually requires some loss in diagnostic sensitivity, but this is not a problem if surveillance is done routinely because the next sampling will reveal the infection.
This discussion presents work done by our group on the performance of the PRRS OF ELISA (IDEXX Laboratories Inc.) using routine diagnostic submissions from expected PRRSV-negative commercial sites. Previous work by our group had showed that the test provided excellent diagnostic sensitivity and specificity under experimental conditions2. In this study, we focused on the performance of the test under field conditions and provided estimates of diagnostic specificity over a range of alternative cutoffs.
PRRS OF ELISA data from two sets of samples were compiled. (1) 596 OF samples from 12 pigs vaccinated with PRRSV modified-live vaccine and sampled over time post-vaccination under experimental conditions. Testing was done on randomized samples after the termination of the experiment. (2) 1,574 OF samples collected on 94 unvaccinated, PRRSV-negative sites and submitted to the Iowa State University Veterinary Diagnostic Laboratory for testing unrelated to this project. After the requested testing was completed on the individual cases, the samples were assembled, randomized and tested on the PRRS oral fluid Ab ELISA.
The data were analyzed using non-parametrical statistical procedures. Data from Set 1 were used to evaluate kit performance using receiver operating characteristic curve (ROC) analysis. Data from Set 2 were used to evaluate ELISA sample-to-positive (S/P) responses by pig age using Turkey's boxplot and linear regression. In addition, both sets of samples were evaluated in terms of the effect of alternative cut-offs on positivity rates using Cochran's Q (Sets 1, 2).
It is recognized that the PRRS OF ELISA has a wide dynamic response range and values as has high S/P = 13.3 were observed in Set 1 (experimental) samples. Because of this strong S/P response, increasing the test cut-off reduced test diagnostic sensitivity (as expected), but a cut-off of S/P ≥1.0 still produced a diagnostic sensitivity estimated at 96.5 % (Table 1 and Figure 1). Note that higher cut-offs did not improve diagnostic specificity because nearly all negative samples had S/Ps < 0.4 (n = 166). Raising the cut-off from S/P ≥ 0.4 to S/P changed the time at which 50% of the animals (six of 12) were ELISA positive from 10 days to 11 days post exposure.
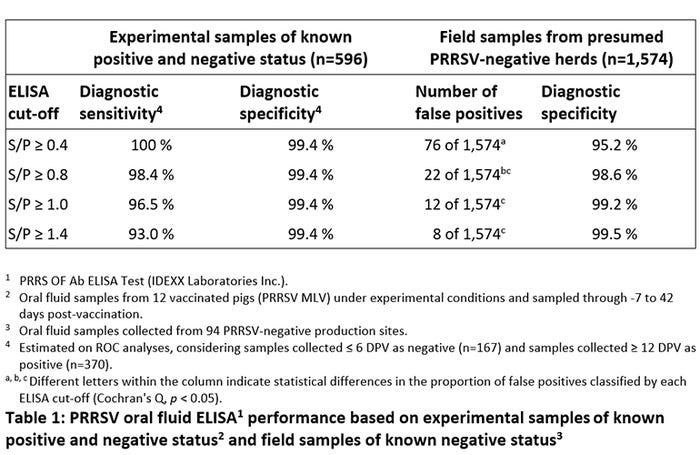
As shown in Table 1, analysis of Set 2 (PRRSV-negative sites) test data show that diagnostic specificity improved from 95.2% at a cut-off of S/P ≥ 0.4 to 99.2% at a cut-off of S/P ≥ 1.0. Figure 2 shows the distribution of PRRSV OF ELISA S/P responses from Set 2 (PRRSV-negative sites) samples. Unexpectedly, non-specific reactions were not random (Figure 2). Rather, they were associated with specific sites, ages (9, 24, 25 weeks of age), and stages (gestation or isolation). The majority of these responses were relatively low (S/P ≤ 1.0) and appear to be relatively transient. The cause(s) of these non-specific responses could not be determined from this study, but is an important area in need of investigation.
Based on the work to date, we recommend using a cut-off of S/P ≥ 1.0 for routine PRRSV surveillance. The data show that this cut-off will improve the diagnostic specificity of the test with minimal impact on diagnostic sensitivity (Table 1) or time to detection. The World Animal Health guidelines state that the same assay may have different cut-offs for distinct applications, e.g., diagnostics versus surveillance. In the case of the PRRSV oral fluid antibody ELISA, the manufacturer's cut-off (S/P ≥ 0.4) is appropriate for diagnostic applications, but a cut-off of S/P ≥ 1.0 provides the higher specificity required for routine surveillance.
Full disclosure of conflict of interest
Jeff Zimmerman has served as a consultant to IDEXX Laboratories Inc. on areas of diagnostic medicine independent of this research. The terms of the consulting arrangement have been reviewed and approved by Iowa State University in accordance with its conflict of interest policies.
References cited
1. Trevisan G, et al. PLoS One. 2019; 14:1-16.
2. Henao-Diaz A, et al. Res Vet Sci. 2019; 125:113-118.
Sources: Alexandra Henao-Diaz, Luis Giménez-Lirola, Phil Gauger, David Baum, Rodger Main, Jeff Zimmerman, Min Zhang, Maria J. Clavijo, Marisa Rotolo and Esteban Ramirez, who are solely responsible for the information provided, and wholly own the information. Informa Business Media and all its subsidiaries are not responsible for any of the content contained in this information asset. The opinions of these writers are not necessarily those of Farm Progress/Informa.
You May Also Like


