The Pigs Are Sick. Is It PEDV?
March 15, 2014

Getting an accurate diagnosis of enteric disease in pigs is getting more complicated since porcine epidemic diarrhea virus (PEDV) has emerged in the U.S. Different diagnostic strategies and testing tools are needed, depending on PEDV infection status and the given situation.
Four common questions regarding PEDV diagnostics fielded by the Iowa State University Veterinary Diagnostic Laboratory include:
1. How do we confirm a diagnosis of PEDV in a naïve (never been infected before) herd?
2. How do we sort out the role of PEDV vs. other causes of diarrhea in a PEDV-positive herd?
3. How can we ensure that new introductions to my herd are not carrying PEDV?
4. How can we monitor fomites (facilities, vehicles, materials) for PEDV?
The specific answers to these questions will depend on the unique situation present on each farm. Swine veterinarians are positioned very well to offer the best strategy for a given situation.
The diagnosis of PEDV when it first enters a naïve population of pigs has been straightforward. Diagnosis is based on the clinical signs (e.g., severe diarrhea in pigs of any age, rapid transmission throughout all pigs on the site, severe mortality in suckling piglets) and detection of PEDV by diagnostic testing. Diagnostic testing simply involves performing a polymerase-chain-reaction test (PCR) on feces from pigs acutely affected with diarrhea. If PEDV is the culprit, the test will be strongly positive and very few samples are required to confirm the diagnosis.
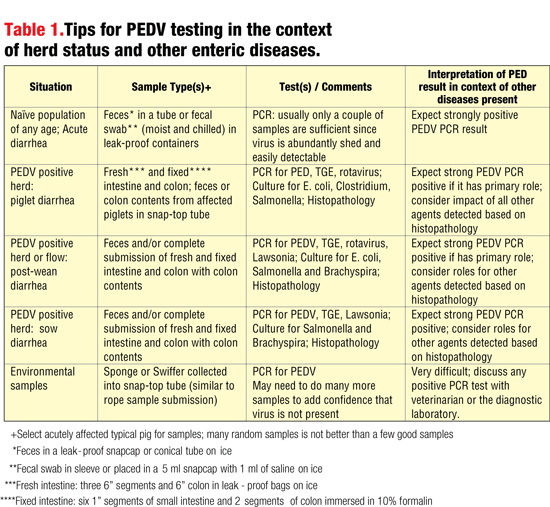
Table 1 offers a very brief summary of submission concepts. More specific information can be found at websites of veterinary diagnostic laboratories, the National Pork Board and elsewhere.
Like what you’re reading? Subscribe to the National Hog Farmer Weekly Wrap Up newsletter and get the latest news delivered right to your inbox every week!
As PEDV becomes more endemic in U.S. herds — that is, when PEDV becomes “seeded down” in a sow farm or flow of pigs — the diagnosis of the role of PEDV vs. other causes of diarrhea is often daunting. In endemic situations, PEDV can clinically appear to be less virulent.
Indeed, across the globe there is diversity in virulence of PEDV such that the disease may be easily confused with less severe or more sporadic enteric diseases (e.g. rotavirus, colibacillosis, Clostridium, Salmonella, Lawsonia or Brachyspira). Each of these agents can cause severe disease on their own; therefore, in PEDV-endemic herds where diarrhea occurs, it is very import to rule out through diagnostic testing the roles for other infectious disease agents.
It is imprudent and costly to automatically assign blame to PEDV when another agent of diarrhea (e.g., Salmonella, Lawsonia, Brachyspira or Transmissible gastroenteritis (TGE)) is a strong contributor to the diarrhea present. Attention to detail will become increasingly important in animal selection, specimen collection and test selection to achieve accurate diagnosis.
Collection of excellent samples for histopathology will be critical to sort out the relative roles and impact of the various pathogens detected by diagnostic testing, particularly in those cases of diarrhea on PEDV-positive farms (see Table 1). If PEDV is the cause of clinical diarrhea, there will be abundant virus in feces, and the PCR result should be unequivocally positive.
A third area for diagnostic testing is a concern for disease risk associated with introduction of animals into a breeding herd. Naïve sow farms will want to pull out all stops to prevent entry of PEDV with scrupulous and consistent execution of details of external biosecurity. The concepts of segregation, isolation and diagnostic testing are wholly appropriate for preventing introduction of PEDV, as well as a host of other diseases. During the isolation period, any diarrheic feces detected can be submitted for PCR testing to rule out the presence of acute PEDV infection.
Examples of tools to monitor for acute or previous infection while pigs are in isolation include one or more of the following:
1. Aggressive testing of any clinical diarrhea that occurs during the isolation period. PCR for PEDV on diarrheic feces is an obvious procedure, but you may want to include PCR or culture for other enteric agents of concern such as TGE, Lawsonia, Salmonella or Brachyspira.
2. Routine surveillance testing using PCR for PEDV on representative oral fluids collected from an isolation facility. Oral fluids have been shown to be useful to detect subclinical infections.
3. Serology is quite useful to determine if an animal has been previously infected. Consult with your local veterinarian for test types (e.g., IFA or ELISA) and availability. A negative result in animals >8 weeks of age suggests no previous infection with PEDV. Serology for PEDV is likely a part of an overall monitoring program for other diseases you wish to exclude from the sow herd.
Another quite different and distinct PEDV testing strategy applies to “environmental” samples. These are samples from transportation vehicles, animal lairage facilities or other surfaces. Samples are collected and tested in an attempt to determine if they are contaminated with PEDV. The mindset for sampling, testing and test interpretation is quite different for this sample type, versus feces or tissues from pigs.
Generally, “swiffers,” or sponges moistened with sterile saline, are used to “mop” suspicious surfaces. Fluid is then squeezed from the material into a snap-cap tube, chilled and submitted for PEDV testing by PCR. Since these samples are not expected to contain nearly as much virus as feces from clinically affected pigs, the interpretation of the test result is more difficult. Consult with your veterinarian or diagnostic laboratory for best practices for sampling, submitting and test interpretations.
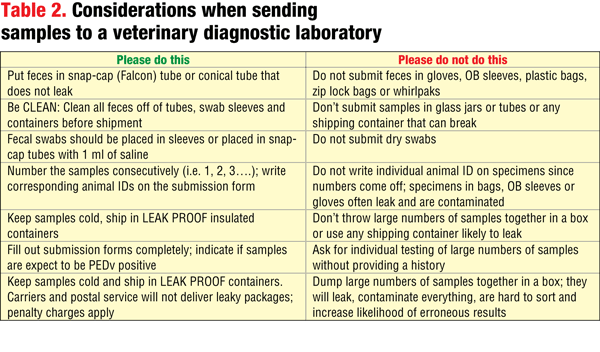
There are huge differences in the amount of PEDV expected to be present in different sample types. Virus concentration ranges from 100 billion genetic copies/mL in feces from suckling piglets to perhaps 10 copies/mL in an environmental sample. Diagnostic laboratories receive hundreds of samples each day. Your diligence in proper collection and packaging of samples is very important in preventing cross-contamination or erroneous results. Some tips for submission can be found in Table 2.
You might also like:
Many Factors are Working Together to Impact Pork Supply
You May Also Like



