Exposing Sows to PEDV to Build Herd Immunity
March 13, 2014

Editor’s note: The following expanded Veterinarians On Call column is devoted to suggestions for exposing a sow herd to porcine epidemic diarrhea virus (PEDV).
We believe, regardless of the following words offered, that no one knows or can accurately predict how present clinical responses to porcine epidemic diarrhea virus (PEDV) infections will manifest over time. There are no tested, objective protocols. There are, at best, vague indices which are often used to define “success” or “failure.” Moreover, variation is a consistent feature of biology and swine production. Herd exposure to farrowing materials has evolved on farms to be a procedure commonly performed without consideration of context, clarity of objectives or process design.
Based on our education and experience, we believe the following represents a logical, empirical, reasoned and balanced approach for herd exposure to PEDV.

What are the goals for controlled oral exposure (feedback) of PEDV?
The primary outcomes to achieve with intentional and controlled exposure of the sow herd to PEDV are:
1. Establishing herd immunity to minimize the time period of baby pig deaths by infecting and recovering all sows and gilts in as short a time frame as possible
2. Ensuring all sows and gilts are on the same timeline to immunity and cessation of shedding of PEDV within the population
Here’s what this process is, using science and experience:
To rapidly achieve herd immunity, it is necessary that EACH and EVERY sow and gilt on the premises be infected to develop active immunity. Therefore, initial outbreaks in naïve herds are managed by whole-herd exposure to virus-containing fecal material from affected pigs by a swift and consistent method to ensure exposure, infection and immunity.
Because PEDV is a mucosal (gut surface) pathogen, it is important to understand that effective active immunity occurs at the level of the gut mucosa in the form of lactogenic immunity provided by the lactogenic IgA antibody in milk.
Ideally, the lactogenic IgA antibody will be sufficiently present in milk throughout lactation to protect piglets from the severe effects of disease. This is in contrast to many diseases in which the IgG antibody of colostrum provides systemic protection for the piglet. Serum antibody and colostrum antibody are less important in providing protection to piglets than is the IgA secretory antibody found in milk.
PEDV is not like porcine reproductive and respiratory virus (PRRS), porcine circovirus type 2 (PCV2), swine influenza virus (SIV) or pseudorabies (PRV), and any extrapolations from knowledge or experiences with other diseases should be made with care. The immune response of the sow is determined by route of exposure, and likely by the magnitude of the dose of PEDV she receives. In adult animals, mucosal immunity with strong IgA stimulation is best stimulated by delivery of a high dose of virulent virus to small intestinal enterocytes via the oral route.
Like what you’re reading? Subscribe to the National Hog Farmer Weekly Wrap Up newsletter and get the latest news delivered right to your inbox every week!
These two factors (high dose and oral route) are even more important when oral exposure is used to try to bolster immunity in a partially immune animal. One large dose, properly applied, is expected to be much more effective in stimulating or bolstering a mucosal immune response than would multiple smaller doses, or the improper application of agent or antigen.
A simple explanation of the objective of exposure in sows can be viewed at the bottom of page 24.
Ongoing disease protection in suckling piglets depends on two major factors:
1. The continuous flow of IgA-laden milk through the piglet intestine
2. The absence of infective doses of PEDV in the environment
These are the basis for two key action items after whole-herd exposure is complete. Firstly, agalactia, or piglets’ inability to nurse, will render them susceptible to infection if PEDV remains in the environment — even at low dose — because there is insufficient IgA intake to neutralize the virus. If one or a few piglets do not nurse and become infected, they will replicate and shed PEDV in high doses, escalating the cycles of endemic infection.
Therefore, aggressive management of piglet milk intake and early humane culling of compromised piglets is warranted. Secondly, after the initial whole-herd exposure, it becomes necessary to eliminate PEDV from the environment. This is accomplished by repetitive, scrupulous and ongoing management and hygiene. Re-establishing a culture of detailed attention to sanitation and disinfection of the farrowing environment specifically, and entire sow farm generally, has potential to eliminate PEDV from the premises. In summary, once herd immunity is established by whole-herd exposure, PEDV elimination from the environment by sanitation and the aggressive management of compromised piglets becomes high priority.
Specific strategies for effective herd immunity:
■ Expose each sow’s gut to virulent PEDV in sufficient concentrations to cause infection, replication and a strong mucosal immune response; therefore, higher doses of virus in inoculum are desirable.
■ Expose all breeding animals within short time frame.
■ The best opportunity for an effective immune response is in naïve animals with no pre-existing immunologic “memory.”
■ The narrow window of exposure establishes a projection-to-immunity calendar for prognostic purposes.
■ Implement a post-exposure strategy to accurately and objectively monitor immune response, viral presence and PEDV-associated pathology.
Why baby pig feces collected immediately at onset of diarrhea are the ideal material for sow and gilt exposure:
PEDV concentration (dose) is very important in stimulating or bolstering immune responses in adult animals. Virus concentration is much higher in suckling piglet feces than in intestines of dead piglets or feces from sows. In fact, the greatest virus concentrations and quantities of feces can be collected in the first 18 hours of clinical signs from young (2- to 5-day-old) piglets.
Why?
■ Neonatal pigs have very long villi with much higher populations of mature intestinal enterocytes, which are most permissive for PEDV replication (see Figures 1 and 2).
■ In contrast, post-weaned and adult pig intestines contain far fewer permissive mature enterocytes, so the total amount of virus that can be produced is much less than in neonates.
■ Infected enterocytes fill with viral particles until they explode, shedding huge numbers of virus particles in liquid feces.
■ Live neonates with diarrhea will shed most of the virus prior to death. Polymerase-chain-reaction (PCR) testing demonstrates virus concentrations to be 10,000 times higher* in piglet feces than in sow feces. That means that 1 gallon of piglet feces has the same number of virus particles as 10,000 gallons of sow feces.
■ Most of the infectious virus generated with PEDV in neonates is shed via fecal excretion in the first 12-18 hours of diarrhea, with less virus remaining at the time of death.
■ Enterocytes and the virus are sloughed and expelled in feces. Intestines of piglets at death have much less virus remaining in infected enterocytes and feces than in feces of acutely affected neonatal piglets (see Figures 3 and 4 on page 25).
Suckling piglets have greater risk for severe disease for several very important reasons:
1. More susceptible cells.
■ The villi of the small intestine of neonates are very long, with many more mature or permissive enterocytes available to be infected by virus.
2. They heal more slowly.
■ The regeneration time for new epithelial cells (enterocytes) produced in the crypt epithelium is longer in neonates than in older pigs.
3. Immature homeostasis.
■ The colon of neonates is functionally immature and less able to compensate for acid-base and electrolyte imbalances, or to resorb water.
4. Osmotic pressures from undigested milk
■ Damage to epithelium reduces enzyme activity and disrupts digestion of milk, which increases the osmotic pressures within the intestine, effectively sucking water out of the piglet’s body and into the intestine and feces.
How can baby pig feces be efficiently collected?
Since feces from live neonates with diarrhea likely contain 10,000 times more virus/unit of volume than sow feces or intestines from dead neonates, piglet feces becomes the ideal material for controlled exposure to the sow herd. Piglet fecal materials can be collected in multiple ways without the need to collect viscera from dead or euthanized piglets. Collecting viscera is time-consuming and provides unnecessary fodder for the scrutiny of public perceptions.
Some example collection methods include:
■ Paper towels, to soak up feces from the floor/mats, can be placed in pens in gestation.
■ Liquid feces can be poured from floor mats, strained and delivered by trigger sprayer on the nose of each sow.
■ Piglets can be placed in carts during the acute phase and diarrhea collected on absorbent material.
■ Diarrhea can be gently expressed and collected in a container by abdominal palpation.
■ Other innovative methods also exist.
What are additional benefits of feces over macerated viscera for caretakers and farm operations?
Farrowing house attendants readily participate in the early identification of illness and collection of quality inoculum. Euthanasia and evisceration of piglets is, rightly, extremely disconcerting to many of these caretakers.
The caretakers’ role is crucial.
Intense focus of caretakers on the following critical areas provides efficiencies, resulting in a rapid and high-dose herd exposure within a short window of time.
■ Identification. Recording or sow card marking of each ill (e.g., anorexia, vomiting, diarrhea) sow and gilt is made, thus documenting effective infection.
■ Material. Virus-concentrated fecal collection is augmented and optimized by the identification of litters as they begin to have diarrhea, and by collection of fecal material from acutely ill piglets.
■ Euthanasia. Piglets can be identified for euthanasia in a more timely manner, i.e., 12-18 hours before they are suffering in extremis. It is worth noting that anticipation of the necessity to euthanize terminally ill piglets humanely and promptly has been much appreciated by staff during an acute PEDV outbreak.
■ Exposure quality assurance. Careful individual exposure of sows and gilts can be conducted throughout the workday by specifically charged teams of people skilled at animal observation. Because duration of illness may be brief in adults, repeated inspection throughout the workday best identifies all affected animals most accurately.
■ Sanitation. Cleanliness, sanitation and disinfection measures can begin immediately following whole-herd exposure in preparation for the recovery phase post-infection.
Reducing environmental load of virus is very important, as exposure to a high concentration of virus may overwhelm the IgA protection provided by milk.
■ Personnel. The time and labor associated with viscera collection and maceration is better applied to animal care. Additionally, the aesthetics of using fecal-only inoculum are much more palatable, which is important for all personnel working with the animals.
■ Other risks. Risks of transmission of other non-enteric pathogens (e.g., influenza, PRRS, many others) are reduced by avoiding viscera maceration processes and procedures.
■ Our best. Practices and methods are based on best medical care, evidence-based medicine justifications, animal welfare considerations and industry image.
How is fecal material best handled to ensure a viable and adequate concentration of virus for exposure?
■ Handling all materials as cold solutions preserves virus viability. Handling fecal material at room temperature may not inactivate all viable, infectious virus, but may lower the concentration.
■ Keep material cold by refrigeration or placing on ice trays. If dilution is necessary, use cold 0.9% saline solution (1 teaspoon of salt in 1 gal. water, refrigerated).
■ Strain through a screen strainer to provide a fluid that can be delivered by trigger sprayer or syringe technique to deliver to the oral cavity of sows and gilts. This virus replicates in small-intestine enterocytes.
■ Refrigerate any material that is not for immediate use.
* For a specific test and agent, the Ct values are rough estimates of relative numbers of genomic copies/unit of sample, and are likely proportional to the number of infectious virions as well. A difference of approximately 3.3 in Ct values is roughly a 10-fold difference in genomic copies. For example, a Ct of 10.0 from piglet feces from acute diarrhea would be expected to have 10 times more genomic copies than a Ct of 13.3, and would have 10,000 times more genomic copies per volume than a Ct of feces from a sow with a Ct of 23.5.
Figure 1: Intestinal Villi from Normal Neonatal Pig
Figure 1 shows the intestinal villi from a normal neonatal pig. Note that the villi are very long, with large surface areas to absorb nutrients and water. The crypts are where new cells are produced which will eventually slide up and replace the cells on the villi. Villi are covered with absorptive epithelium (arrow). Crypts are where replacement cells are produced (oval).
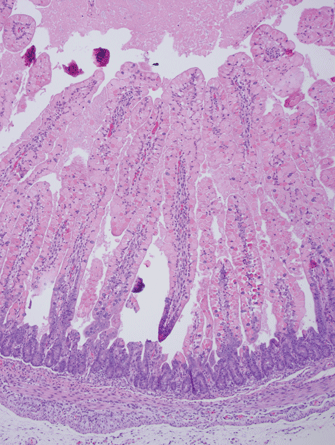
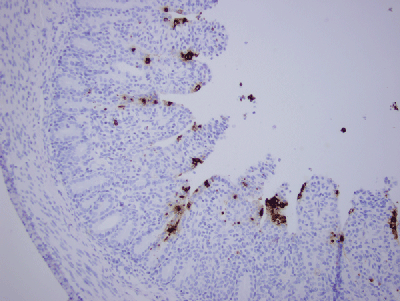
Figure 2: Early PEDV Infection in a Neonatal Pig
This figure shows early PEDV infection in a neonatal pig. The brown stainings are infected cells lining the villi in a neonatal piglet roughly eight hours after infection. All of the brown cells will die, releasing billions of virus particles to infect more cells and more pigs. Villi are covered with infected epithelial cells stained brown. Crypt epithelium is spared and will be the source of new cell growth.
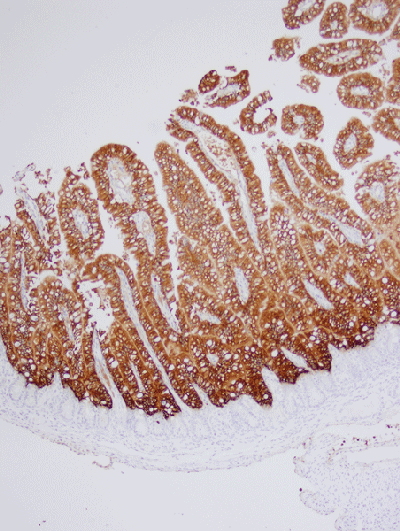
Figure 3: Neonatal Pig 36 Hours After PEDV Infection
This figure shows the severe decrease in the height of villi (villus atrophy) and loss of absorptive epithelium approximately 36 hours after infection of a neonatal pig. There is much less surface area to absorb nutrients or water. The crypts are increasing in length as they produce more cells to rebuild the absorptive cells of the villi.
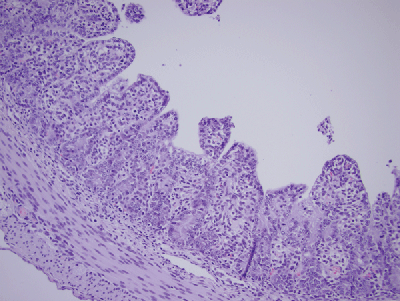
Figure 4: Severely Damaged Neonatal Pig Gut Post-PEDV Infection
This figure shows just a few brown-staining PEDV-infected cells remaining in the gut of a neonatal piglet roughly 36 hours after infection. Note that there is very little absorptive surface remaining since most of those cells have been destroyed by PEDV.
Collaborators on this column include:
Kent Schwartz, DVM, MS
Iowa State University Veterinary Diagnostic Laboratory, Ames IA;
Steve Henry, DVM, Dipl. ABVP, Lisa Tokach, DVM, Dipl. ABVP, and Megan Potter, DVM, Ph.D., Abilene Animal Hospital PA, Abilene, KS;
Don Davidson, DVM, MS and Cody Egnor, DVM, PFFJ LLC, Taylor, AZ.
You May Also Like



