USDA joins FDA in cell-based meat product oversight
FSIS will oversee the production and labeling of human food products derived from the cells of livestock and poultry.
March 7, 2019
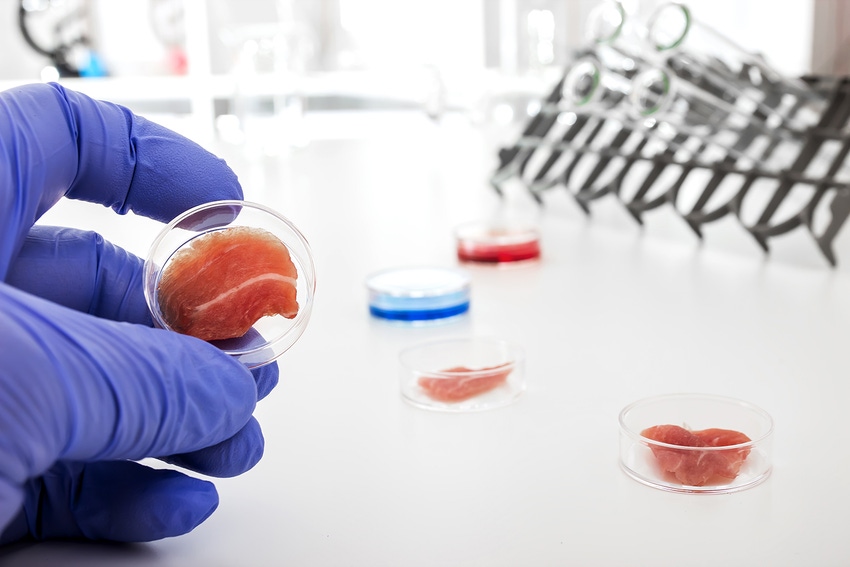
The USDA Food Safety and Inspection Service is now part of the cell-based meat products supervision process. FSIS and the FDA have announced a formal agreement to jointly oversee the production of human food products derived from the cells of livestock and poultry.
“Consumers trust the USDA mark of inspection to ensure safe, wholesome and accurately labeled products,” says USDA Deputy Under Secretary for Food Safety Mindy Brashears. “We look forward to continued collaboration with FDA and our stakeholders to safely regulate these new products and ensure parity in labeling.”
The formal agreement describes the oversight roles and responsibilities for both agencies and how the agencies will collaborate to regulate the development and entry of these products into commerce. This shared regulatory approach will ensure that cell-cultured products derived from the cell lines of livestock and poultry are produced safely and are accurately labeled.
“We recognize that our stakeholders want clarity on how we will move forward with a regulatory regime to ensure the safety and proper labeling of these cell-cultured human food products while continuing to encourage innovation,” says Frank Yiannas, FDA Deputy Commissioner for Food Policy and Response. “Collaboration between USDA and FDA will allow us to draw upon the unique expertise of each agency in addressing the many important technical and regulatory considerations that can arise with the development of animal cell-cultured food products for human consumption.”
Under the formal agreement, the agencies agree upon a joint regulatory framework wherein FDA oversees cell collection, cell banks, and cell growth and differentiation. A transition from FDA to FSIS oversight will occur during the cell harvest stage. FSIS will oversee the production and labeling of human food products derived from the cells of livestock and poultry.
On Oct. 23-24, 2018, FSIS and FDA held a joint public meeting to discuss the use of cell culture technology to develop products derived from livestock and poultry. The public meeting focused on the potential hazards, oversight considerations and labeling of cell cultured food products derived from livestock and poultry.
Sources: USDA and FDA, who are solely responsible for the information provided, and wholly owns the information. Informa Business Media and all its subsidiaries are not responsible for any of the content contained in this information asset.
About the Author(s)
You May Also Like


