FDA announces FY 2020 animal drug user fee rates for ADUFA and AGDUFA
In the period since Congress first passed ADUFA in 2003, the FDA has approved more than 300 new animal drugs — both original approvals and major enhancements to these approvals.
August 1, 2019
The U.S. Food and Drug Administration announced in the Federal Register today the fiscal year 2020 fee rates and payment procedures for animal drugs subject to user fees under the Animal Drug User Fee Amendments of 2018 (ADUFA IV) and Animal Generic Drug User Fee Amendments of 2018 (AGDUFA III).
ADUFA, originally signed into law in 2003 and reauthorized in 2008, 2013 and 2018, amends the Federal Food, Drug and Cosmetic Act, 21 U.S.C. 321 et seq., and authorizes the FDA to collect fees for certain animal drug applications and supplements, products, establishments and sponsors of animal drug applications and/or investigational animal drug submissions. These resources help support activities undertaken by the FDA to ensure that new animal drug products are safe and effective for animals and that food derived from treated animals will be safe for consumption. ADUFA IV reauthorizes the FDA to collect user fees through FY 2023.
AGDUFA, originally signed into law in 2008 and reauthorized in 2013 and 2018, was designed to enhance the performance of the generic new animal drug review process, which enables the FDA to ensure that generic new animal drug products are safe and effective. AGDUFA III reauthorizes the FDA to collect user fees for certain abbreviated applications for generic new animal drugs, generic new animal drug products, and from certain sponsors of abbreviated applications for generic new animal drugs and/or investigational submissions for generic new animal drugs. The user fees for sponsors are prorated based on the number of approved abbreviated applications the sponsor currently holds. AGDUFA III reauthorizes the FDA to collect user fees through FY 2023.
In the period since Congress first passed ADUFA in 2003, the FDA has approved more than 300 new animal drugs — both original approvals and major enhancements to these approvals (e.g. new indications, new species). Over the nine-year period since AGDUFA was passed, the FDA has approved more than 120 generic new animal drugs.
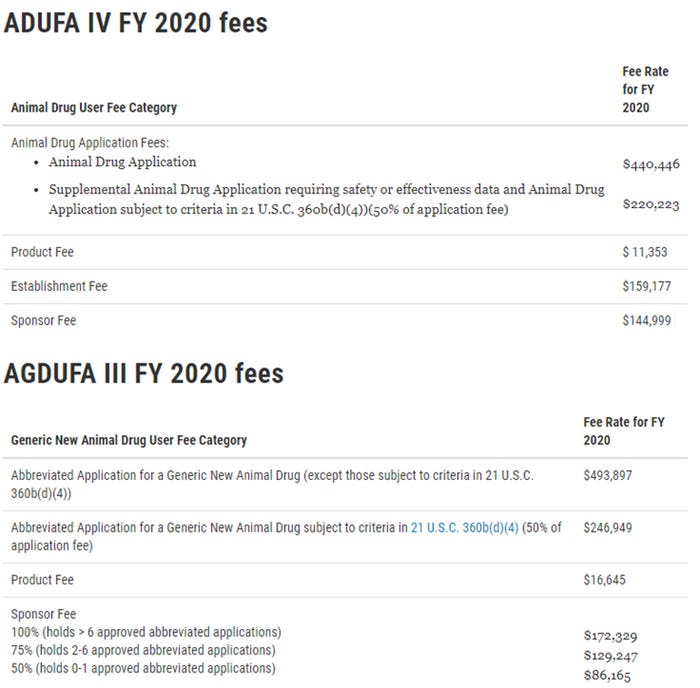
The tables below reflect the ADUFA IV and AGDUFA III fee rates for FY 2020.
In addition to announcing the ADUFA and AGDUFA user fee rates for FY 2020 and explaining how such rates were calculated, the Federal Register notices also provide details on how to submit payment of these fees to the FDA. The application fee rates are effective for applications received by the FDA’s Center for Veterinary Medicine from Oct. 1 until Sept. 30, 2020. The FDA will issue invoices for FY 2020 product, establishment and sponsor fees by Dec. 31, and payment will be due by Jan. 31, 2020. The FDA will not accept an application for filing unless the FDA has received payment for all fees due from the sponsor.
Source: Food and Drug Administration, which is solely responsible for the information provided, and wholly owns the information. Informa Business Media and all its subsidiaries are not responsible for any of the content contained in this information asset.
You May Also Like



