Improving oral fluid-based surveillance - You can help!
Strengths and weaknesses still being tested as it is relatively new concept.
March 1, 2022

In swine medicine, oral fluids are the sample of choice for pathogen detection and routine surveillance. First introduced in 2010, the method is well established in the swine industry because of the advantages it offers over individual pig samples. That is, samples are easily collected with no stress to workers or pigs and, overall, oral fluids are more likely to detect the pathogen of interest and at a lower cost than individual pig samples (Henao-Diaz et al., 2020).
Aggregate testing data from Midwestern Veterinary Diagnostic Laboratories suggest that oral fluids have met an industry need (Figure 1). That is, the number of cases submitted to the VDLs for PRRSV testing that included oral fluid specimens for testing increased from ~5% in 2010 to 37% in 2021. At the same time, number of cases with serum samples decreased from 53% in 2010 to 15% in 2021.
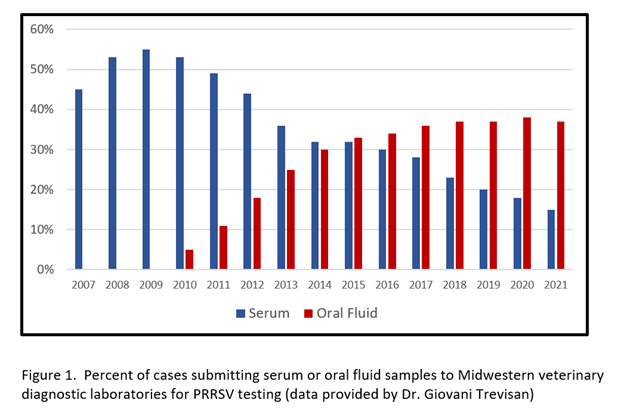
Nevertheless, oral fluid-based surveillance is still a relatively new concept and both field veterinarians and researchers continue work to test the strengths and weaknesses of the approach. Thus, a quick search of the literature for 2020 and 2021 found refereed publications on the detection of a wide variety of viral pathogens of swine in oral fluids, including African swine fever virus, atypical porcine pestivirus, PCV, porcine astroviruses, porcine hemagglutinating encephalomyelitis virus, porcine respirovirus 1, PRRSV, pseudorabies virus, and others.
Of course, the research we do on oral fluid-based surveillance is interesting and even fun! However, it has a serious purpose. Early detection of important pathogens, e.g., African swine fever virus (ASFV), classical swine fever virus (CSFV), foot-and-mouth disease virus (FMDV), and others, is the cornerstone of a decisive and effective control-and-elimination response. But in this we routinely fall short:
In Brazil (1978), an ASFV outbreak misdiagnosed as CSFV in the index farm quickly spread to 11 Brazilian states. Eradication took 8 years and cost ~$20 million USD (Moura et al., 2010).
In the 1997-1998 CSFV outbreak in The Netherlands, a retrospective study found that the virus was actually spreading in the country 5- to 7-weeks before it was recognized (Stegeman et al., 2000). Eradication was successful, but it cost ~$2.3 billion USD (Meuwissen et al., 1999; Stegeman et al., 2000).
In the United Kingdom (2001), early FMDV infections went unnoticed and the virus was distributed widely via the movement of infected animals. Eradication took 6 months, the euthanasia of 4 million animals, and ~$4.0 billion USD (Davies, 2002).
In the U.S., we do not know when porcine epidemic diarrhea virus (PEDV) was introduced, but it spread to at least 12 states within 8 weeks of its initial detection in 2013 (Stevenson et al., 2013; Niederwerder and Hesse, 2018).
The lesson these outbreaks try to teach us is that surveillance needs to be both accurate and timely if we are to match the speed at which infectious agents move between and within countries. Anything less puts our livestock industries at risk. Today, our most pressing problem is the amazing speed with which ASFV is spreading around the globe. A case in point: ASF recently popped up in Italy - thousands of miles from the nearest ASFV cases in Germany. Similarly, ASFV appeared in the Dominican Republic (July 2021) -- and brought the problem to our doorstep. For that reason, the industry continues to explore the use of oral fluids as a diagnostic sample with the potential to be used in surveillance if ASFV or another foreign animal disease enters the U.S. Thus, both the National Pork Board (NPB) and the Swine Health Information Center (SHIC) have funded ASFV research projects in Germany, Romania, Uganda, Vietnam, and elsewhere.
Research outside the U.S. is important, but there is equally important work that needs to be done back here in the U.S. - and you can help! The industry is changing quickly to adapt to a smaller labor force and constantly working to become more efficient. These changes are reflected in the production facilities that are being built, for example, in the size of buildings and the number of pigs per pen. These changes are important to oral fluid surveillance because effective oral fluid sampling relies on understanding pig behavior. Most of the previous work on sampling has been done in pens of 25 pigs; in which case we know that at least 70% of the pigs in the pen will chew on the rope within 30 minutes. But what happens in pens of 50 pigs or 500 pigs? How many ropes should we provide to be sure that a sufficient proportion of the pigs in pen contribute to the sample?
Thus, to adapt oral fluid sampling to a changing industry, we need a better understanding of how the industry is changing, i.e., how many pigs are housed per pen across the industry? Short surveys distributed through the American Association of Swine Veterinarians and the National Pork Board are designed to help gather information on pen sizes and drive our future research. Of course, all responses will be anonymous unless the respondent says otherwise!
Please click the appropriate link below to access the survey. Thank you!
Source: Grzegorz Tarasiuk, Giovani Trevisan, Pam Zaabel, Rodger Main, Jeff Zimmerman, who are solely responsible for the information provided, and wholly owns the information. Informa Business Media and all its subsidiaries are not responsible for any of the content contained in this information asset.
References
Davies G. 2002. The foot and mouth disease (FMD) epidemic in the United Kingdom 2001. Comp. Immunol. Microbiol. Infect. Dis. 25, 331-343.
Henao-Diaz A, Giménez-Lirola L, Baum DH, Zimmerman J. 2020. Guidelines for oral fluid-based surveillance of viral pathogens in swine. Porcine Health Management 6:28.
Meuwissen MP, Horst SH, Huirne RB, Dijkhuizen AA. 1999. A model to estimate the financial consequences of classical swine fever outbreaks: Principles and outcomes. Preventive Veterinary Medicine 42:249-270.
Niederwerder MC, Hesse RA. 2018. Swine enteric coronavirus disease: A review of 4 years with porcine epidemic diarrhoea virus and porcine deltacoronavirus in the United States and Canada. Transboundary and Emerging Diseases 65:660-675.
Moura JA, McManus CM, Bernal FEM, de Melo CB. 2010. An analysis of the 1978 African swine fever outbreak in Brazil and its eradication. Revue Scientiique et Technique 29:549-563.
Stegeman A, Elbers A, de Smit H, Moser H, Smak J, Pluimers F. 2000. The 1997-1998 epidemic of classical swine fever in the Netherlands. Veterinary Microbiology 73:183-196.
Stevenson GW, Hoang H, Schwartz KJ, Burrough ER, Sun D, Madson D, Cooper VL, Pillatzki A, Gauger P, Schmitt BJ, Koster LG, Killian ML, Yoon KJ. 2013. Emergence of porcine epidemic diarrhea virus in the United States: clinical signs, lesions, and viral genomic sequences. Journal of Veterinary Diagnostic Investigation 25:649-654.
You May Also Like



