Senecavirus A: diagnosis and current U.S. situation
Circulation of SVA on farms could interfere with diagnosis of other foreign animal diseases and affect economic trade.
February 23, 2021

Senecavirus A (SVA) cause vesicular disease (VD) and epidemic transient neonatal losses (ETNL) in swine. Vesicular lesions associated with SVA are clinically indistinguishable from those observed in foot-and-mouth disease (FMD), vesicular stomatitis (VS), swine vesicular disease (SVD), and vesicular exanthema of swine (VES), which are considered exotic diseases in the United States. Seroprevalence studies indicate that SVA is endemic and highly prevalent in the United States, and it might circulate subclinically in sows and finisher pigs. It has also been shown that rodents play an important role in the ecology of SVA. In addition, persistent infection studies suggest that subclinically infected animals play an essential role in carrying and maintaining the virus in endemic farms. Current epidemiological data support that occasional outbreaks still occur and cause a multifactorial economic burden to the swine industry.
Clinical outbreaks of SVA VD are characterized by an abrupt onset of vesicles that progress to ulcerative dermatitis on the snout, oral cavity, or coronary bands, which affect a high percentage of sows and finisher pigs. During acute outbreaks, the main clinical manifestations are anorexia and lameness. A variable number of animals go off-feed first due to a short and transient fever and later due to vesicles in the oral cavity that can rupture. Vesicles in the coronary band cause pain and lameness that reduce motility and access to the feeders. The number of animals affected during acute breaks in naïve farms varies from 10–90%. Although a high number of animals could be affected, the disease is not associated with increased mortality.
Outbreaks of ETNL produce 30–70% mortality in piglets between one and four days of age without specific or characteristic clinical signs. Reported clinical signs from farms that have suffered this condition are unspecific and include weakness, salivation, skin rash, neurologic signs, diarrhea, and sudden death. These clinical signs linger for 3–10 days before ceasing in surviving piglets. Lesion observed during necropsy and histological evaluation are unspecific and include interstitial pneumonia and ballooning degeneration of the urinary bladder and renal pelvis epithelium, atrophic enteritis of the small intestines, non-suppurative meningoencephalitis. Skin lesions on neonatal animals are rare but, when present, are similar to those reported in adults.
The factors that may predispose farms to different clinical presentations have not been completely elucidated and are likely multifactorial. Moreover, farms that report clinical VD outbreaks do not necessarily present ENTL or vice versa. Interestingly, both clinical presentations are self-limiting and only last for one or two weeks. It has also been demonstrated that not all infected animals develop vesicular lesions during clinical outbreaks; indeed, subsets of animals can remain subclinical within clinically affected herds. It has been reported that clinical signs can persist for months in swine operations having continuous pig flow in the growing site (i.e., not all in-all out by site), or in farrow-to-finish sites.
Diagnosis
Clinically, vesicular lesions are indistinguishable from other vesicular diseases of swine. Therefore, clinical diagnosis has to be complemented with direct (virus isolation, detection of viral RNA) and/or indirect (antibody) detection of the pathogen. Etiological diagnosis can be achieved by qRT-PCR and virus isolation from vesicular material. However, samples have to be carefully selected.
Fluid from acute vesicles proves to be the best diagnostic specimens for virus isolation (VI) and PCR. Rupture and dried vesicles with long environmental exposure of the ulcerated areas can lead to false negatives. In situ detection can be achieved by directly detecting viral antigen or genetic material by immunohistochemistry (IHC) or in situ hybridization (ISH). Skin biopsies of the margins of ruptured vesicles are also desired specimens for IHC or ISH's etiological diagnosis. Antibody detection methods currently available include indirect immunofluorescence assay (IFA), virus neutralization assays, competitive enzyme-linked immunosorbent assays (cELISAs), and indirect ELISAs targeting different viral proteins.
For serological diagnosis, paired samples from a subset of affected animals at two-week intervals would be sufficient to make the etiological diagnosis. Antibodies have a long-lasting presence and single time point’s samples would not be sufficient to evaluate acute exposure. However, this is still a valuable tool to determine the presence of the virus at the farm. The ISU Veterinary Diagnostic Laboratory has developed an oral fluid ELISA that allows determining the presence of specific SVA antibodies in saliva.
Still, it has been demonstrated that animals can shed the virus and develop SVA-specific antibodies without evidence of clinical disease, suggesting that the virus may be circulating subclinically. Subclinically infected pigs can experience transient viremia, and most importantly, shed virus in feces, oral fluids, and nasal secretions, perpetuating the virus in the environment. Our studies demonstrated that humoral immunity against SVA after the experimental challenge lasted approximately 60 days, and reinfection after antibodies disappear, results in a recurrence of clinical outbreaks of VD. However, most farms that reported SVA clinical outbreaks have not reported VD's recurring outbreaks under field conditions. This may indicate the development of herd immunity through continuous low-dose exposure to the virus.
Experimental studies showed that SVA can be shed for up to 35 days post-infection, while, in some animals, despite lack of viremia and viral detection in feces or oral fluids, the virus can be harbored in the tonsils for up to 60 days post-infection.
Vertical transmission from sows to their piglets also occurs, but lactogenic immunity might prevent clinical disease in neonatal piglets. However, neonatal piglets might become subclinically infected and carry the virus into the nursery, increasing viral transmission and exposure during commingling.
Current situation in the United States
Since 2015, several outbreaks of VD and ETNL associated with the presence of SVA have been reported in multiple swine-producing countries, including the United States, Brazil, Canada, China, Colombia, Thailand, Mexico, and Vietnam. With cases appearing in new locations each year, SVA appears to be on the path to global prevalence. Seroprevalence studies in the U.S. revealed that SVA is endemic and highly prevalent, affecting 75.8% of sows (Figure 1) and 42.7% of grower-finisher herds (Figure 2). The same studies showed a wide geographical distribution. The frequency of grower-finisher farms with a within-herd prevalence ranging from 1% to 50%, from 51% to 70%, and from 71%–100% was 70.8%, 5.5%, and 1.8%, respectively. While the percentage of sow farms with a within-herd prevalence ranging from 1% to 50%, from 51% to 70%, and from 71% to 100% was 58.2%, 10.98%, and 18.68%, respectively. In addition to these serological studies, SVA has been detected in healthy finishers and culled pigs in transit to the slaughterhouse, indicating that SVA is circulating subclinically in swine herds.
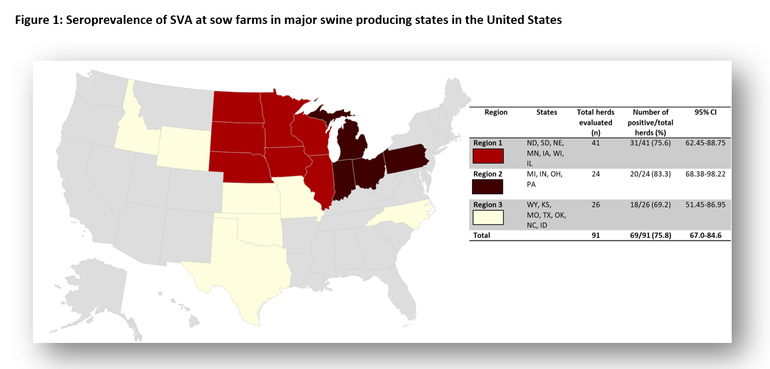
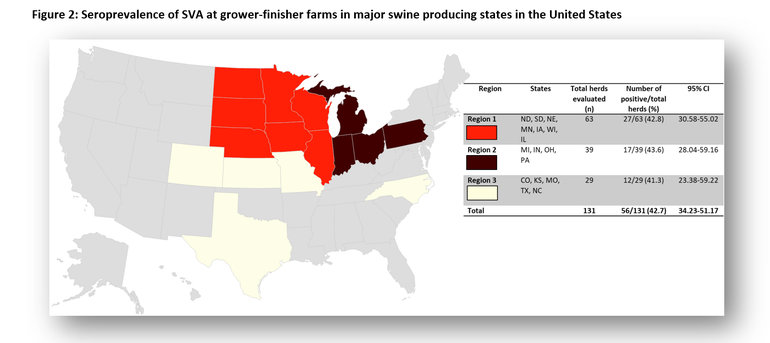
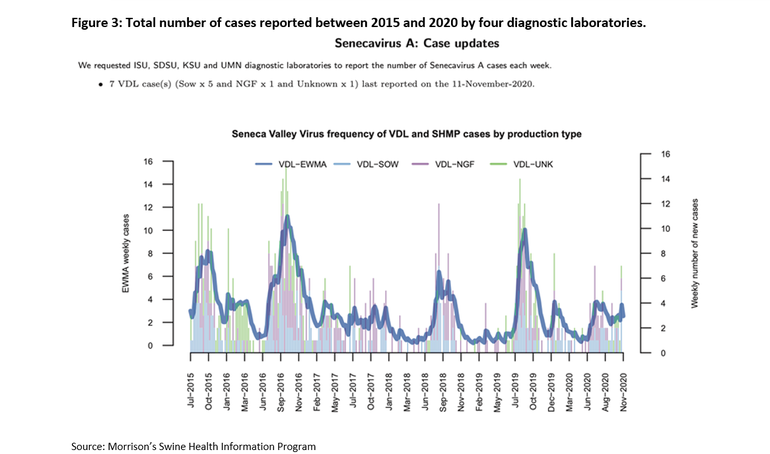
Another piece of interesting information related to the current SVA situation can be found in a compiled case report from multiple diagnostic laboratories (ISU, SDSU, KSU, and UMN). The compiled information shows that there is a seasonal pattern associated with positive cases. An increment in the frequency of SVA-positive cases has been consistently reported during the summer months, regardless of the sample matrix evaluated, including different pig and environmental samples in sow, nursery-grower-fisher farms (Figure 3).
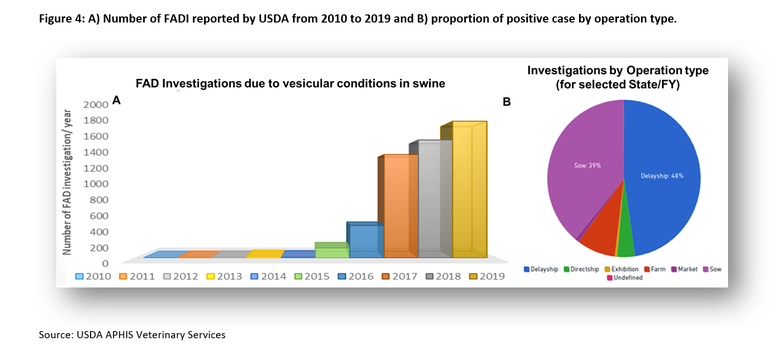
USDA reports show that the number of swine foreign animal diseases (FAD) investigations for VD’s have increased dramatically during the last four years. The main driver of this increment seems to be associated with an increased detection/circulation of SVA since the previous re-break in 2015. Besides, this survey shows that sow farms are the second most important stage/operation type within the production chain where these FAD investigations take place only after butcher hog plants for animals that pass through secondary markets [Delayship] (Figure 4) (data provided by USDA APHIS Veterinary Services).
The role of federal laboratories in confirming the presence of SVA and ruling out the potential threats of other foreign vesicular diseases has been critical for the U.S. swine industry. However, the economic cost incurred by FAD investigations has not been fully estimated. The cost of each outbreak for the producers is associated with laboratory testing and losses related to the relocation of farm and employ resources, treatment costs, increased number of days to slaughter, and the increased feed conversion/reduction on feed intake, closure of slaughter plants/delay in processing during slaughterhouse breaks, cost- and risk associated with live-pigs exports.
Thus, SVA is still circulating clinically and subclinically in the United States swine populations. Even though the vesicular form of SVA is transient and clinical outbreaks have high morbidity, low mortality, SVA-associated vesicular lesions are still a challenge for the swine industry. Circulation of SVA on swine farms could interfere with the diagnosis of other foreign animal diseases and affect economic trade.
Highlights:
SVA prevalence is high in sow and grower-finisher farm across the United States.
SVA farm diagnoses require a combination of direct and indirect techniques.
The virus can be detected for short periods in serum to long periods in oral secretions and feces.
SVA antibody can be detected in serum and oral fluids.
Sows farms can be persistently infected and the virus can perpetuate in sows herds.
SVA has driven a dramatic increase in the number of FADI investigations.
The total cost of SVA outbreaks and investigations needs to be further evaluated.
References:
Houston E, Giménez-Lirola LG, Magtoto R, Mora-Díaz JC, Baum D, Piñeyro PE. Seroprevalence of Senecavirus A in sows and grower-finisher pigs in major swine producing-states in the United States. Preventive Veterinary Medicine. 2019;165:1-7.
Gimenez-Lirola, L.G., Rademacher, C., Linhares, D., Harmon, K., Rotolo, M., Sun, Y., Baum, D.H., Zimmerman, J., Piñeyro, P., 2016. Serological and Molecular Detection of Senecavirus A Associated with an Outbreak of Swine Idiopathic Vesicular Disease and Neonatal Mortality. J Clin Microbiol 54(8), 2082-2089.
Guo, B., Piñeyro, P.E., Rademacher, C.J., Zheng, Y., Li, G., Yuan, J., Hoang, H., Gauger, P.C., Madson, D.M., Schwartz, K.J., Canning, P.E., Arruda, B.L., Cooper, V.L., Baum, D.H., Linhares, D.C., Main, R.G., Yoon, K.J., 2016. Novel Senecavirus A in Swine with Vesicular Disease, United States, July 2015. Emerging Infectious Diseases 22(7), 1325-1327.
Maggioli, M.F., Fernandes, M.H.V., Joshi, L.R., Sharma, B., Tweet, M.M., Noll, J.C.G., Bauermann, F.V., Diel, D.G., 2019. Persistent Infection and Transmission of Senecavirus A from Carrier Sows to Contact Piglets. J Virol 93(21).
Sources: Pablo Piñeyro, Luis Gimenez-Lirola, Giovani Trevisan, Daniel Linhares, Iowa State University, who are solely responsible for the information provided, and wholly own the information. Informa Business Media and all its subsidiaries are not responsible for any of the content contained in this information asset.
You May Also Like



