Tissues, blood, feces and serum have historically been the samples needed to perform most diagnostic tests. But in the last few years, oral fluids have proven to be useful samples for detecting certain pathogens circulating in groups of pigs.
February 4, 2013

Tissues, blood, feces and serum have historically been the samples needed to perform most diagnostic tests. But in the last few years, oral fluids have proven to be useful samples for detecting certain pathogens circulating in groups of pigs.
Validated tests for oral fluids at the Iowa State University Veterinary Diagnostic Laboratory (ISUVDL) include polymerase chain reaction (PCR) for detection of genetic material (RNA or DNA) of porcine reproductive and respiratory syndrome (PRRS) virus, swine influenza virus A (SIV), Mycoplasma pneumonia and porcine circovirus type 2 (PCV2), as well as the detection of antibodies for PRRS virus using the X3 enzyme-linked immunosorbent assay (ELISA) test or indirect fluorescent antibody test (IFA) for both European and North American strains.
Importantly, positive results from oral fluid testing should not be used for disease diagnosis, but rather interpreted simply as surveillance testing for the presence of the target pathogen within the population being tested.
Experience and data (not shown) suggest that properly collected and submitted oral fluids are a more sensitive and more economical sampling strategy than serum for the detection of PRRS virus and SIV in a population, with added benefits of labor savings and being an animal-friendly procedure.
Many other targets have been detected or explored in oral fluids, including genetic material or antibodies of infectious agents (e.g. Mycoplasma hyorhinis, Lawsonia intracellularis, Salmonella, Haemophilus, Erysipelothrix) as well as pharmaceutical agents (antibiotics). Validation for many of the test targets is still in progress, therefore those tests may not be routinely offered by diagnostic laboratories. Interpretation of positive results should be carefully considered for those targets for which test validation is lacking.
Using Oral Fluids to Detect PRRS Virus
The success rate of either PRRS virus isolation or sequencing is directly correlated to the concentration of the virus in the sample. Oral fluids collected from populations generally have less concentrations of virus than are found in serum from the viremic pigs. This is partly because oral fluids are pooled samples more often used for surveillance or monitoring larger populations when clinical disease is not apparent, whereas, serum is often collected from pigs with clinical signs of disease. As a consequence, historical success rates for PRRS virus sequencing or isolation from oral fluids has been lower from oral fluid samples than from serum samples.
Sequencing of PRRS virus, PCR-positive samples is a tool for monitoring the movement and diversity of PRRS virus in a farm, production system, or larger geographic area or region over time. As Figure 1 shows, the number of PRRS virus ORF5 sequencing analyses conducted at the ISUVDL has increased by more than 300% during 2009-2012, while the average turnaround time has been cut in half.
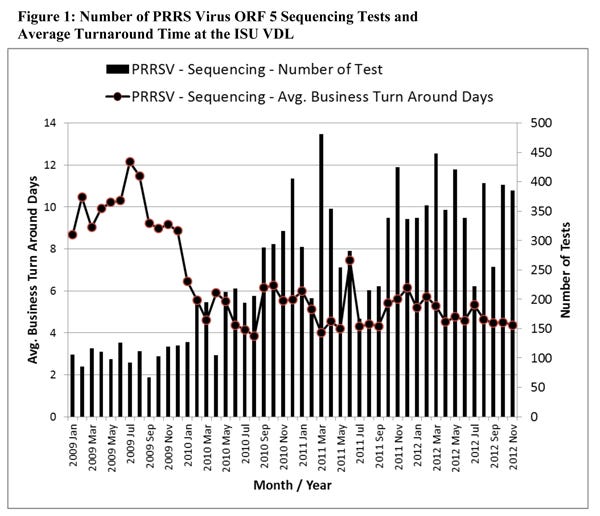
The faster turnaround time is because of a more seamless PRRS virus ORF5 sequencing procedure with specialized technical staff solely committed to conducting sequencing analyses on a daily basis. The increase in PRRS virus ORF5 sequencing requests coincides with the increased use of oral fluids samples, particularly for PRRS virus surveillance and monitoring.
The success rate of sequencing PRRS virus from oral fluids can be markedly improved by:
1. Attention to details in collecting oral fluids to minimize amounts of feces or feed in the samples;
2. Immediate chilling (or freezing) of the oral fluid sample at collection and maintaining the “cold chain” through the entire submission process; and
3. Choose the samples with the highest concentration of virus for sequencing. Currently, data (not shown) from the ISUVDL suggests that sequencing success from serum and oral fluids is fairly similar if starting concentration is similar. A similar relationship exists for PRRS virus isolation.
Researchers at ISUVDL adapted the PRRS virus X3 oral fluid ELISA test from the IDEXX HerdCheck X3 ELISA test to reliably detect antibodies in oral fluids, with excellent sensitivity (94%) and nearly 100% specificity. Two major considerations for interpretation of positive results are:
1. Maternal antibody can be detected in piglets up to 5-6 weeks of age; and
2. PRRS virus antibody that is present in feed or water (e.g. feeding of porcine plasma protein) is detected. Therefore, it is prudent to not use oral fluid PRRS virus X3 ELISA in groups of pigs consuming such products.
Detection of SIV from Oral Fluids
Oral fluids have been an excellent tool for detection of SIV by PCR in populations of pigs. This virus apparently circulates more frequently and widely amongst pigs of all ages than perceptions of clinical signs would imply. SIV shedding can be quite transient (2-3 days) and can occur at low doses when endemic in a herd.
It is quite difficult to identify and sample those individual pigs that are currently shedding SIV with just nasal swabs or tissues. For example, collection of oral fluids in late suckling stage or early postweaning pigs often identifies SIV as a likely contributor in cases where pigs do not start well or have a cough. Once detected by PCR, subtyping can usually be accomplished to determine if the virus is H1N1, H1N2, H3N3 or H3N1.
Further characterization of SIV from oral fluids via sequencing or isolation is more difficult because of naturally occurring inhibitors in oral fluids. This relationship is similar to PRRS virus in that these techniques are more successful when samples are properly handled (kept clean and cold) and concentrations of virus are higher.
Detection of Mycoplasma from Oral Fluids
Detection of Mycoplasma pneumonia from oral fluids by PCR is useful to confirm that mycoplasma is present and/or circulating within a population. But a positive test doesn’t confirm mycoplasma as the cause of disease. As is true with virtually all diagnostic tests, a negative result does not prove that the agent is not present in the population. But a positive test can reliably be interpreted that the population is positive for the presence of the organism.
Detection of PCV2 from Oral Fluids
Oral fluids can be used to detect PCV2 by PCR. Since PCV2 is a very common infection and persists for long periods in the environment, positive results may require careful interpretation.
Oral Fluid Submission Guidelines
A brief review of oral fluid collection techniques and tips on collection and test interpretation can be found at http://vetmed.iastate.edu/vdpam/disease-topics/oral-fluids. Briefly, they include:
Collect oral fluid samples from pigs (hang cotton ropes for 20 minutes). Prevent ropes from contacting the floor or feces as much as possible.
Wring the rope into a plastic bag, collect the fluids and submit to the laboratory in 5-ml Falcon “snap-cap” tubes.
Immediately chill samples and keep them cold or frozen until they reach the lab.
Take Home Messages
The applications of oral fluids in diagnostic tests have increased substantially in the past 2-3 years.
PRRS virus ORF5 sequencing requests on oral fluid samples has increased exponentially as the number of larger-scale PRRS virus control projects (production-system specific or area/regional control) has increased.
Although PRRS virus ORF5 sequencing success rates in oral fluids are lower than rates achieved with serum and/or tissue samples, this appears related to the concentration of detectable PRRS virus in diagnostic specimens. PRRS virus PCR-positive serum, tissue and oral fluid samples from clinically affected animals have markedly higher concentrations of detectable PRRS virus than oral fluid surveillance samples.
Careful collection (minimal fecal exposure), maintaining the cold chain of oral fluid samples (up to and including freezing upon collection) and reducing the time from collection to testing are interventions that appear to improve PRRS virus ORF5 sequencing outcomes in oral fluid samples.
You May Also Like



