Denagard® and CTC are proven to work together to provide whole-herd protection, reducing disease incidence and improving overall performance, including heavier final weight and hot carcass weight.
July 26, 2018

Sponsored Content
When it comes to treating disease on your operation, isolating the source can be difficult. That’s why protecting the entire population with Denagard® is the proactive choice that’s right for your herd and best for your business.
Denagard and chlortetracycline (CTC) control swine dysentery associated with Brachyspira hyodysenteriae susceptible to tiamulin, treats swine bacterial enteritis caused by Escherichia coli and Salmonella choleraesuis sensitive to CTC, and treats bacterial pneumonia caused by Pasteurella multocida sensitive to CTC.
Denagard and CTC both act at the ribosomal level, inhibiting protein synthesis, but their binding sites are different. Together, the two antibiotics increase the activity against labeled pathogens and enlarge the spectrum of control to certain gram-negative bacteria. While using Denagard alone does not require a Veterinary Feed Directive (VFD), using Denagard + CTC does.
Study - Effects of two Denagard + CTC pulses in grow-finish pigs1
To understand the effects of Denagard + CTC in grow-finish pigs, a commercial population of approximately 1,150 growing pigs averaging approximately 75 lb with a known history of swine pneumonia and bacterial enteritis was used. Confirmation of the disease-causing bacterial agents (Pasteurella multocida, Escherichia coli, and Salmonella choleraesuis) was determined using laboratory diagnostics.
Treatment groups
Treatment 1 was the control group, with no medication provided. The pigs in Treatment 2 were given Denagard at 35 grams/ton + 400 grams/ton CTC from days 7 to 20 and days 49 to 62.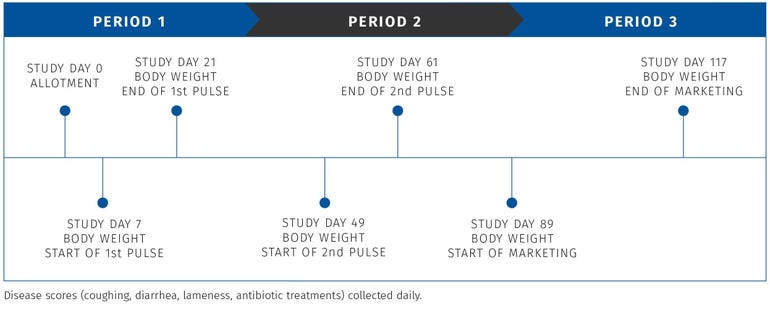
Key findings
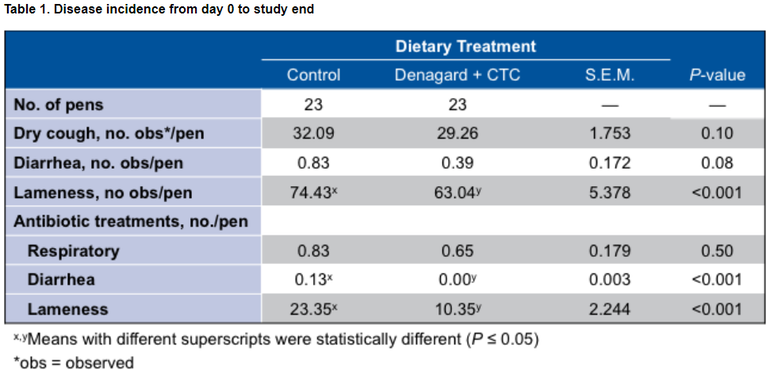
Incidence of disease for pens treated with Denagard + CTC was numerically lower than in the control group. Additionally, the need to treat diarrhea and lameness with antibiotics was reduced for those in the Denagard + CTC group.
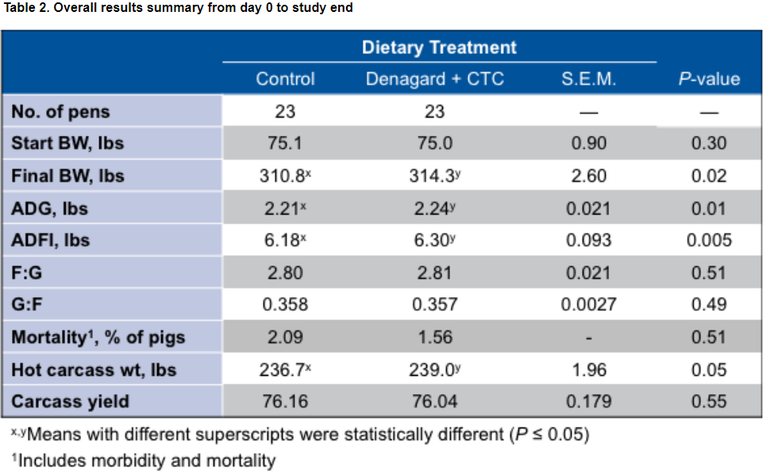
As a result of controlling and treating disease, pens treated with Denagard + CTC performed better overall than the control group with:
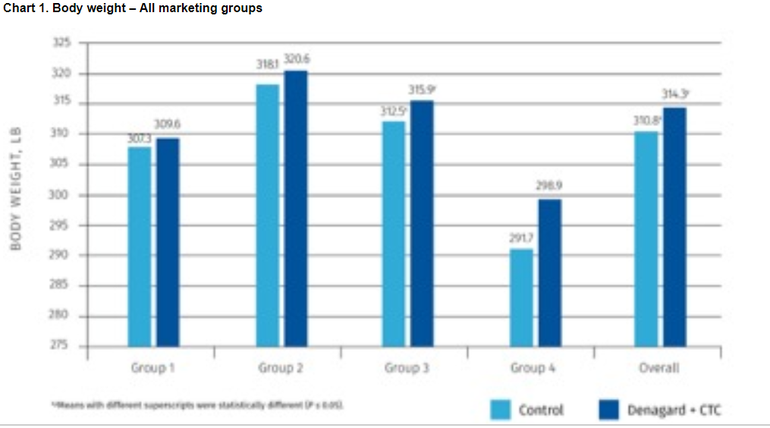
● Heavier final weights — 314.3 lb vs. 310.8 lb
● Better average daily gain (ADG) — 2.24 lb vs. 2.21 lb
● Improved average daily feed intake (ADFI) — 6.30 lb vs. 6.18 lb
Heavier hot carcass weight (HCW) — 239.0 lb vs. 236.7 lb
Are you doing what’s right for your herd and best for your business?
Talk to your local Elanco sales representative or technical consultant to learn more about protecting whole-herd health with Denagard.
The label contains complete use information, including cautions and warnings. Always read, understand and follow the label and use directions.
Denagard® LC
INDICATIONS:
For treatment of swine dysentery associated with Brachyspira hyodysenteriae susceptible to tiamulin:
Utilize Denagard LC in drinking water at 3.5 mg/lb (60 ppm) for five consecutive days
For treatment of swine pneumonia associated with Actinobacillus pleuropneumoniae susceptible to tiamulin:
Utilize Denagard LC in drinking water at 10.5 mg/lb (180 ppm) for five consecutive days
IMPORTANT SAFETY INFORMATION
Swine being treated with Denagard (tiamulin) should not have access to feeds containing polyether ionophores (e.g. lasalocid, monensin, narasin, salinomycin and semduramicin) as adverse reactions may occur.
If signs of toxicity occur, discontinue use of medicated water and replace with clean, fresh water.
Withdraw medicated water 3 days before slaughter after treatment at 3.5 mg/lb and 7 days before slaughter following treatment at 10.5 mg/lb.
Keep out of reach of children. Avoid contact with skin.
For use in drinking water of swine only. Prepare fresh medicated water daily. The effects of tiamulin on swine reproductive performance, pregnancy and lactation have not been determined.
If no response to treatment is obtained within 5 days re-establish the diagnosis.
Denagard 10 Premix
INDICATIONS:
For control of swine dysentery associated with Brachyspira hyodysenteriae susceptible to tiamulin:
Feed 35 g/ton
Feed continuously as sole ration
2-day withdrawal
For treatment of swine dysentery associated with Brachyspira hyodysenteriae susceptible to tiamulin:
Feed 200 g/ton
Feed for 14-days for treatment
7-day withdrawal
For control of ileitis associated with Lawsonia intracellularis susceptible to tiamulin:
Feed 35 g/ton
Feed for not less than 10 days
2-day withdrawal
IMPORTANT SAFETY INFORMATION
Swine being treated with Denagard (tiamulin) should not have access to feeds containing polyether ionophores (e.g. lasalocid, monensin, narasin, salinomycin and semduramicin) as adverse reactions may occur.
If signs of toxicity occur, discontinue use.
Withdraw 7 days before slaughter at 200 g/ton and 2 days before slaughter at 35 g/ton.
Keep out of reach of children. Avoid contact with skin.
For use in swine only.
The effects of tiamulin on swine reproductive performance, pregnancy and lactation have not been determined.
Denagard + CTC
INDICATIONS:
For the control of swine dysentery associated with Brachyspira hyodysenteriae susceptible to tiamulin and for treatment of swine bacterial enteritis caused by Escherichia coli and Salmonella choleraesuis sensitive to chlortetracycline and treatment of bacterial pneumonia caused by Pasteurella multocida sensitive to chlortetracycline:
35 g/ton of Denagard + 400 g/ton (10 mg/lb body weight in daily divided doses) CTC
Feed for 14 days
2-day withdrawal
Using Denagard alone does not require a Veterinary Feed Directive (VFD). Using Denagard + CTC does require a VFD.
CAUTION: Federal law restricts medicated feed containing this veterinary feed directive (VFD) drug to use by or on the order of a licensed veterinarian.
IMPORTANT SAFETY INFORMATION
Swine being treated with Denagard (tiamulin) should not have access to feeds containing polyether ionophores (e.g. lasalocid, monensin, narasin, salinomycin and semduramicin) as adverse reactions may occur.
If signs of toxicity occur, discontinue use.
Withdraw 7 days before slaughter at 200 g/ton and 2 days before slaughter at 35 g/ton.
Keep out of reach of children. Avoid contact with skin.
For use in swine only.
The effects of tiamulin on swine reproductive performance, pregnancy and lactation have not been determined.
1Elanco Animal Health. Data on file.
Denagard and Elanco are trademarks of Elanco or its affiliates.
© 2018 Elanco or its affiliates.
Elanco supports the use of shared class antibiotics for therapeutic uses while under the oversight of a veterinarian. More details on Elanco’s Antibiotic, Welfare and Sustainability Policies can be found on https://www.elanco.com/our-responsibilty#responsible-use-of-antibiotics.
fdhlth 10969-1 | USSBUDEN00028
About the Author(s)
You May Also Like



