October 1, 2017
Sponsored Content
Key ingredients and nutritional regimens that elevate maternal circulating insulin-like growth factor-1 have proven to elicit beneficial effects on birth weight, muscle fiber number and sow body condition. These benefits are established before and just after birth through programming of cellular metabolism, and influence the animal’s lifetime growth and productivity. This article helps clarify how IGF-1 influences the rate and efficiency of lean meat production through its influence on both fetal muscle cell development and neonatal muscle cell growth.
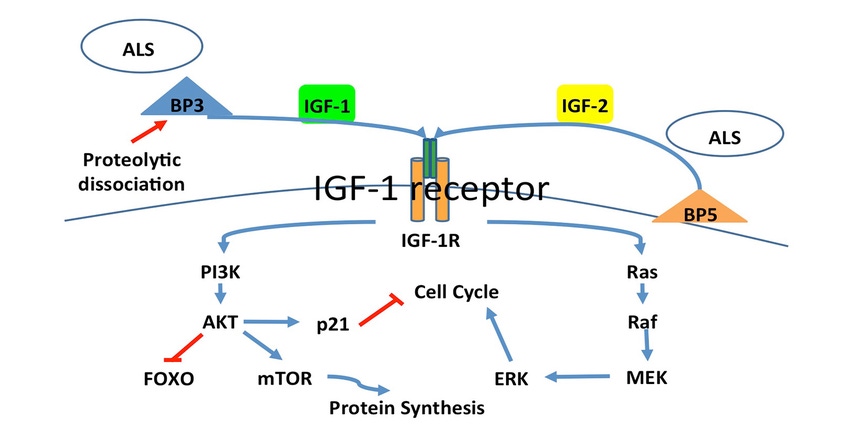
Recently, Musser (2017) explained how IGF-1 receptor signaling plays a key role in musculoskeletal development. Insulin-like growth factor-1 can stimulate muscle hypertrophy (increased cell size) and hyperplasia (increased cell number) through two distinct pathways. The phosphatidylinositol-3 kinase pathway stimulates muscle hypertrophy, while the mitogen activated protein kinase pathway stimulate muscle hyperplasia (Engert et al., 1996; Schiaffino and Mammucari, 2011;Figure 1).
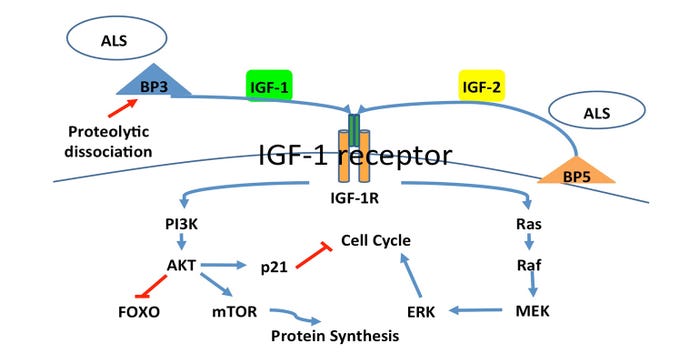
Figure 1: Insulin-like growth factor-1 receptor binding triggers the phosphatidylinositol-3 kinase pathway, or mitogen activated protein kinase pathway stimulating muscle hypertrophy or hyperplasia, respectively (Engert et al., 1996; Schiaffino and Mammucari, 2011).
During early to mid-gestation, hyperplasia establishes the number of muscle cells and stem cells available for postnatal growth. Throughout the last quarter of gestation and postnatal growth, hypertrophy increases muscle cell size and controls ultimate muscle mass.
betaGRO® is a commercially available product that can increase circulating IGF-1 levels. Recently, a whole system approach to evaluating the programming effects of betaGRO® was conducted. Offspring from sows supplemented betaGRO® during gestation produced hot carcasses that tended to be 4.4 pounds heavier (P = 0.07) when compared to controls (Figure 2).
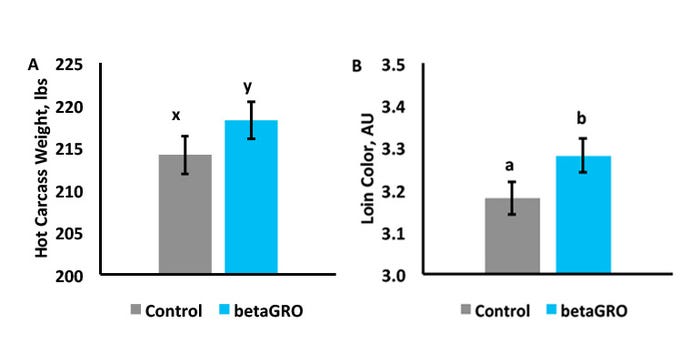
Figure 2: A) Hot Carcass Weight. B) Loin muscle National Pork Producers Council color score.
a, bMeans with differing superscripts differ (P<0.05).
x, yMeans with differing superscripts differ (p<0).
In addition, there was an increase (P = 0.01) in loin redness score of offspring from betaGRO®-supplemented sows compared to control sows. Therefore, these betaGRO®-induced [C1] color changes may be associated with changes in muscle fiber composition.
While these production parameters were improved, they do not explain how betaGRO® stimulated these effects. Therefore, tissue culture methodology was employed to assess the effects of betaGRO® on muscle progenitor cell activity (Khatri et al., 2017). Muscle progenitor cells were isolated from fetuses at Day 60 of gestation and from neonatal piglets within 24 hours of birth. Day 60 of gestation was chosen to model the programming effects during development of secondary muscle fibers, while 24 hours after birth was chosen to model the rapid muscle growth that occurs during neonatal life. Proliferation assays were conducted to assess treatment effect on cell doubling, while fusion and myotube width assays were conducted to assess treatment effect on protein synthesis.
In fetal cells, betaGRO® stimulated 19% more muscle progenitor cells to proliferate compared to negative control cells. Compared to the positive control, cells administered betaGRO® were 83% as proliferative (P < 0.01; Figure 3).
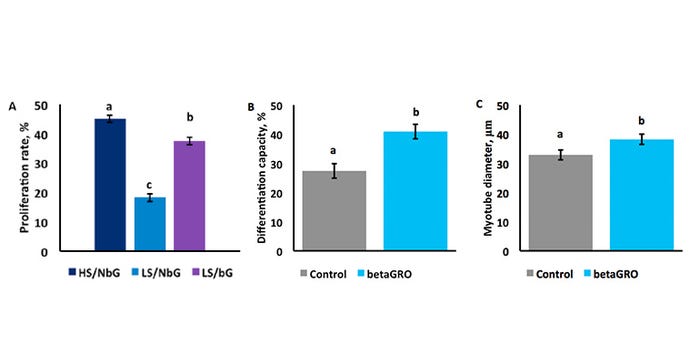
Figure 3: A) Day 60 muscle progenitor proliferation. B) Neonatal muscle progenitor myotube fusion. C) Neonatal muscle progenitor myotube growth.
a,b,cMeans with differing superscripts differ (P < 0.01).
This demonstrates that even in the suboptimal conditions of the negative control treatment, betaGRO® improved muscle progenitor cell hyperplasia to almost the response seen with the ideal conditions experienced by the positive control. Biologically, this may likely lead to an increase in muscle fiber and/or progenitor cell establishment.
In neonatal cells, betaGRO® supplementation increased the fusion of muscle progenitor cells into myotubes by 33% and increased myotube width by 14% (P < 0.01). These measures are both associated with increased protein synthesis. These divergent effects compared to the fetal cell data suggests betaGRO® promoted phase specific muscle development and growth. These tissue culture studies help explain the positive performance benefits observed in offspring lifetime performance when betaGRO® is fed to the sow.
Implications: Maximizing muscle development and metabolic efficiency is crucial to the profitability and sustainability of pork production. Nutrition and health regimens that optimize lean pork production should maximize muscle cell proliferation during gestation, and hypertrophy after birth, to achieve performance and economic benefits.
Engert, J. C., E. B. Berglund, and N. Rosenthal. 1996. Proliferation precedes differentiation in IGF-I-stimulated myogenesis. J. Cell Biol. 135:431-440.
Khatri, O. S., M. A. Vaughn, K. J. Phelps, J. M. Gonzalez. 2017. In vitro betaGRO® supplementation stimulates myogenesis of porcine fetal myoblasts and porcine satellite cells in a divergent manner. National Animal Science Meeting, Baltimore, MD. 317. doi:10.2527/asasann.2017.317.
Musser, R. 2017. Research on fetal programming covers multiple disciplines. Feedstuffs. 89: 30-31, 39.
Schiaffino, S., and C. Mammucari. 2011. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet. Muscle. 1:4. doi:10.1186/2044-5040-1-4.
Co-authored by:
John Michael Gonzalez, Ph.D., Associate Professor Animal Sciences and Industry, Kansas State University
Mathew A. Vaughn, Ph.D., Research Associate, Puretein Bioscience LLC
Joe Loughmiller, Ph.D., Vice President, Puretein Bioscience LLC
Robert Musser, Ph.D., Director of Tech Service, NutriQuest
About the Author(s)
You May Also Like



