Pharmacokinetics allows researchers to determine the movement of drugs through the pig.
April 19, 2017

By Locke A. Karriker, DVM, M.S., and diplomate of the American College of Veterinary Preventive Medicine, Iowa State University
The description of how drugs move through the pig or sow is referred to as pharmacokinetics (PK). Discovering the pharmacokinetics of a drug provides two very important pieces of information. First, PK can explain how much of the drug gets to where it is needed, for instance in the lungs for a respiratory infection. Secondly, PK studies can reveal how much of the drug remains in the animal as time passes so that marketing and consumption can be planned for a time after the drug is sufficiently removed from the animal.
The PK characteristics of a drug are influenced by individual animal differences in metabolism, hydration and health, among other things. Until recently, the implications of these differences for treatment efficacy and the potential for residues were relatively unknown in swine, and very little research was available to help veterinarians and caretakers maximize drug efficacy while minimizing the potential for residues.
Recently, work at Iowa State University and elsewhere has begun to describe the effects of some of these influences on PK, with implications for both drug efficacy and residue avoidance at marketing. This work is applicable and important for field situations because most drug studies used to develop treatment regimens, withdrawal periods and regulatory approval are conducted on clinically healthy animals that are not consistent with the animals that need treatment in the field.
Four studies are described here that evaluate the influence of concurrent viral infection, the benefit of vaccination, the persistence of drug in tissues, and the potential for cross-contamination between treated and untreated animals via the environment. The first study evaluated the changes in ceftiofur PK due to porcine reproductive and respiratory syndrome virus and Streptococcus suis co-infection. The second compared ceftiofur PK when pigs were infected with PRRSV both with and without prior vaccination. The third and fourth studies looked at the amount of time necessary to eliminate penicillin and ampicillin from cull sows to avoid residues at marketing and the risk of detecting contamination in nontreated animals housed with treated animals.
None of the doses or treatment regimens described are intended to serve as recommendations for treatment or meant to represent common practices in the field. Producers should consult with their veterinarian for guidance on what treatment regimens are legal and effective. The dose regimens used in these studies were selected to investigate drug, animal and disease interactions within the constraints of proper research study design.
Study 1: Co-infection with PRRSV and Streptococcus suis lowered ceftiofur drug concentrations in the serum and increased the clearance rate of drug from the pig.
This study, published in the Journal of Veterinary Pharmacology and Therapeutics (2015 Oct; 38(5):475-81. doi: 10.1111/jvp.12209) determined the impact of porcine reproductive and respiratory syndrome virus and Streptococcus suis co-infection on the pharmacokinetic profile of ceftiofur hydrochloride in pigs after intramuscular injection. To do this, 18 clinically normal crossbred gilts were assigned by weight into a challenge group (10 pigs) or a control group (eight pigs). The challenge group was inoculated with 2 milliliters of a wild-type PRRSV isolate intranasally (Pictured below) and eight days later inoculated with a virulent strain of Streptococcus suis.
When the first signs of clinical disease appeared, the pigs in both groups received a single intramuscular injection of ceftiofur hydrochloride at a 5 milligrams per kilogram bodyweight dose. Serial blood samples were then collected to characterize the plasma concentration over time curve. Co-infected pigs demonstrated less total amounts of drug exposure in the blood and lower peak concentrations of drug in the blood as well as faster rates of clearance, indicating drug kinetics were altered by infection. Since there are legal restrictions on changing the dose of ceftiofur to compensate for these changes, this study implies that understanding and reducing co-infections, including viruses which are not the targets of antimicrobials, has the potential to make treatment regimens more effective for the bacterial infections that are a target of antimicrobials. Proper placement of vaccination and prevention strategies for viruses might have the added value of preserving the efficacy of antimicrobial drugs when they are needed. This leads to the question of whether interventions directed toward PRRSV co-infections could preserve ceftiofur PK and, therefore, efficacy in pigs.
Study 2: Vaccination does mitigate the negative impact of PRRSV infection on the pharmacokinetics of ceftiofur in pigs.
Several studies like the one just described at Iowa State University and elsewhere have demonstrated that a PRRSV wild-type infection could influence antimicrobial drug PK even though antimicrobials do not work directly against viruses. The hypothesis is that infection and associated immune responses influence drug PK rather than a direct interaction between viruses and the drugs.
To determine if interventions aimed at mitigating the clinical impact of PRRS virus would protect against these changes in PK, another study was performed. Also reported in the Journal of Veterinary Pharmacology and Therapeutics (2016 Nov 24. doi: 10.1111/jvp.12369), this study had two main objectives:
■ determine if PRRS modified live virus vaccine alone impacts the pharmacokinetic profile of ceftiofur crystalline-free acid in pigs,
■ determine if PRRS MLV vaccination prevents PK impacts when vaccinated pigs are challenged with a wild-type PRRS virus
To do this, 38 clinically healthy, PRRS virus naïve barrows were allotted to one of four groups:
Group A. Control (received no vaccine and no wild-type PRRS virus challenge)
Group B. Vaccinated only
Group C. Challenged with wild–type virus only
Group D. Vaccinated followed by wild-type challenge.
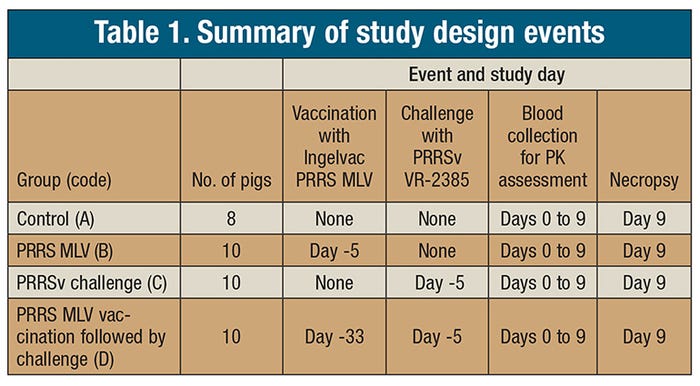
After groups B and D were vaccinated (Ingelvac PRRS MLV vaccine [Boehringer Ingelheim Vetmedica]), groups C and D were intranasally challenged with PRRS virus VR-2385. At time 0, all groups were intramuscularly administered ceftiofur crystalline-free acid (CCFA) at a 5 mg/kg of body weight dose. Blood was sampled at time 0 (immediately pretreatment), 0.25, 0.5, 1, 6, 12, 24, 48, 96, 144, 192, and 240 hours post-injection. Table 1 describes the trial events for each of the groups.
Plasma was analyzed for ceftiofur and related metabolites using liquid chromatography coupled with mass spectrometry. The resulting plots of concentration in the plasma versus time post-injection were used to mathematically model PK parameters describing the movement of the drug in the pigs.
Control pigs (Group A) and the vaccine-only pigs (Group B) were not significantly different from any evaluated PK parameter, suggesting that vaccine alone did not alter the PK of ceftiofur in these pigs. When compared to Group A, pigs that were infected with wild-type virus (Group C) had less total drug exposure in the blood and faster drug clearance, which was consistent with previous studies. When pigs were vaccinated before the wild-type challenge (Group D), they had PK parameters that were more consistent with pigs in Groups A and B and were statistically different from the values of the wild-type-only infected pigs (Group C).
In this study, it appears that vaccination alone with a modified live vaccine does not influence PK parameters of CCFA. When challenged, previous vaccination prevented changes to PK that were observed in wild-type PRRS virus infections, suggesting that treatment and vaccination programs might be coordinated to improve treatment outcomes.
Study 3: Procaine penicillin G takes at least 44 days to clear from kidneys and injection sites on cull sows.
Procaine penicillin G is labelled for the treatment of erysipelas caused by Erysipelothrix rhusiopathiae in swine. It is a broad-spectrum antibiotic effective against gram-positive and gram-negative aerobes. For this reason, penicillin G is occasionally considered for extra-label treatment of a wide range of swine pathogens in sow herds where approved options do not exist. Relying on older PK data generated when drug detection technology was not as sensitive as that employed now created a situation where dosing regimens based on the old data increased the risk of a detectable residue in the present screening system at slaughter. Basically, newer drug detection technology is much more sensitive, measuring what happens when the drug reaches very low concentrations in the pig. In the past, these levels were not measurable, and projections or assumptions about how the last amounts of drug were removed from the body were used. Now that more sensitive detection technology is available, these assumptions can be replaced by direct measurement.
This project determined the tissue, plasma and urine residue depletion of procaine penicillin G in cull sows at label and extra-label doses, with corresponding calculation of the number of days it would take for tissue levels to fall below certain detection levels. It also examined the utility of urine, plasma and environmental samples as matrices for residue detection and the potential for environmental contamination that might be picked up by another animal that was not treated. Penicillin is excreted primarily in the urine, which creates a question about potential exposure for untreated pen mates.
Forty-seven sows were assigned to one of three treatment groups receiving 3,000 international units per pound of bodyweight, intramuscularly, once a day, procaine penicillin G (n=15) or 15,000 IU per pound of bodyweight, intramuscularly, once a day, procaine penicillin G (n=16), or a negative control group receiving sterile saline injections with no drug (n=16). Sows were sampled at 1, 6, 14 and 28 days post-treatment.
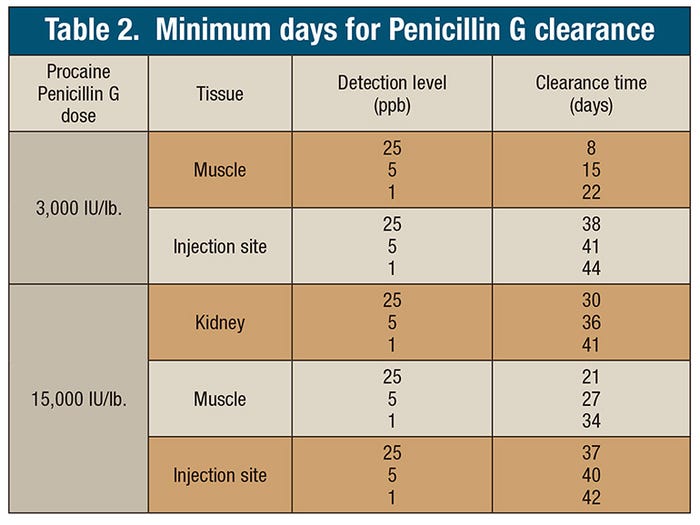
At both treatment levels, the necessary clearance period was calculated for three detection levels (25 parts per billion, 5 ppb and 1 ppb). Table 2 summarizes these clearance periods for muscle, kidney and injection site. With a 25-ppb detection level, clearance from kidney at a dose of 15,000 IU per pound was determined to be at least 30 days. Injection site clearance was estimated to take at least 37 days. Environmental samples were rarely positive, did not correlate with animal status, and were not a good tool for residue detection in the sows as a screening tool. From this study, there is no evidence to support the environmental transfer of penicillin G from treated to untreated sows. Untreated sows were pen housed with treated sows at both dose levels and subjected to the same tissue testing, but remained negative.
Study 4: Ampicillin can take as long as 89 days to clear from injection sites on cull sows.
The tissue residue depletion profile of ampicillin trihydrate in cull sows and the utility of other sampling matrices such as plasma, oral fluids and urine samples was determined in the fourth study.
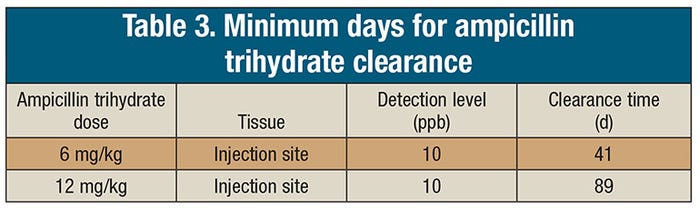
Sixty cull sows were assigned to one of two treatment groups and received a 6 mg/kg (n=30) dose of ampicillin trihydrate or a 12 mg/kg (n=30) dose of ampicillin trihydrate once daily for three days. Three sows from each treatment group were sampled at 1, 3, 5, 8, 10, 12, 15, 20, 30 and 40 days following the final day of treatment. Plasma was analyzed for ampicillin and related metabolites using liquid chromatography coupled with mass spectrometry. Ampicillin residues were not detected in kidneys after Day 1 following treatment. In contrast, when tissues from the injection sites were tested, the drug was found there for the entire duration of the study with residues persisting at 40 days. The injection site location and needle sizes followed industry guidelines. Injection site clearance times were estimated for injection sites at 41 and 89 days for a 6 mg/kg and a 12 mg/kg dose, respectively. Plasma ampicillin residue concentrations were similar to kidney ampicillin residues with most drug cleared before Day 6. Ampicillin residues were not detected in oral fluids samples. Urine samples were generally positive longer than the kidney samples, suggesting that urine from individual sows might be useful for determining if the drug is cleared from the kidneys. However, given the extended period of drug presence at injection sites, a negative test via urine would not confirm that a particular sow was free from drug residues at the injection site.
Although ampicillin and penicillin are drugs from the same antimicrobial class and have efficacy against some of the same diseases, their chemical structures and PK interactions with the sow differ. Penicillin was found to remain in both kidneys and injection sites for extended periods. Ampicillin was found to clear quickly from kidneys but persist at injection sites for periods exceeding those of penicillin in these study conditions.
The reduced use and extended withdrawal periods that have been voluntarily implemented by pork producers using penicillin should be extended to ampicillin products as well. The data also suggest that the pork safety of these products might be improved with further research targeted at understanding why a drug remains at the site of injection.
The studies described above were made possible by funding from the National Pork Board, Boehringer Ingelheim, the Swine Medicine Education Center and the Dr. Douglas and Ann Gustafson Professorship for Excellence in Veterinary Teaching at Iowa State University. Critical to the outcome was the collaboration of peers in academia and industry and the hard work of students, for which the author is very grateful.
You May Also Like



