The clinical signs in pigs infected with vesicular disease caused by SVA are variable and can range from no outward signs, to nonspecific signs such as decreased appetite or fever, or pigs may develop vesicles, or blisters, on the skin or in the mouth.
September 12, 2017

By Matthew Sturos and Fabio Vannucci, University of Minnesota
Senecavirus A (also called Seneca Valley Virus) is a virus that has been circulating in pigs in the United States for at least the past 30 years, based on historical viral isolates, but was only first fully characterized in 2002 as a contaminating virus in cultured cells used in a research laboratory. The name for this virus is derived from Seneca Creek in Maryland, which is nearby the research laboratory that initially characterized the virus. Following its characterization, the virus had been associated with sporadic cases of vesicular disease in Canada and the United States. Beginning in 2014 there were outbreaks of vesicular disease in several countries, including Brazil, the United States, Canada, China and Colombia, in which SVA was detected but no other viruses known to cause vesicular disease were present.
Accompanying the vesicular disease, some affected sow herds also experienced transient increased losses in piglets. These outbreaks of vesicular disease have continued up through 2017, with peaks of reported disease commonly occurring in late-summer and early fall. Early attempts to recreate the vesicular disease in pigs through experimental infection were unsuccessful, but in 2015 multiple research groups were successful in causing vesicles, or blisters, in pigs inoculated with SVA, which has confirmed that this virus can be a cause of vesicular disease in pigs. The role of SVA in the reported cases of transient neonatal losses is still uncertain, as this phenomenon has not yet been reproduced experimentally.
The clinical signs in pigs infected with vesicular disease caused by SVA are variable and can range from no outward signs, to nonspecific signs such as decreased appetite or fever, or pigs may develop vesicles, or blisters, on the skin or in the mouth. Common vesicle locations are on the nose and feet. These delicate vesicles rupture very easily and often the crusted or ulcerated remnants of vesicles are what are initially noticed. Lesions affecting the feet may result in lameness and in severe cases may cause detachment of portions of the sole or hoof wall. These vesicular lesions are indistinguishable from other transboundary vesicular diseases, such as foot-and-mouth disease, and for this reason all cases of vesicular disease require immediate reporting to your state and federal animal health officials for further investigation.
While only sporadic cases had been reported between 1988 and 2014, more than 230 SVA cases have been confirmed after July 2015 in the United States. The similarities among SVA and foreign animal diseases have raised numerous false alarms, and major economic impacts associated with: (1) temporary closure of pork processing plants until the diagnosis of foreign animal diseases has been ruled out, (2) reallocation of resources for diagnostic investigation of FMD and other vesicular diseases, (3) mortality of neonatal piglets and (4) increased culling rates in affected sows. In addition, this scenario has drawn attention from the regulatory authorities due to potential compliances and decreases of diagnostic investigations for foreign animal diseases in SVA cases, as the disease becomes endemic in the U.S. swine population.
The neonatal mortalities associated with SVA infections have been reported in litters of piglets, up to approximately 1 week of age, developing diarrhea and can have increased mortality on sow farms experiencing outbreaks of vesicular disease due to SVA. These piglets often do not have gross or microscopic lesions. SVA has been detected in feces and multiple organs from these piglets by polymerase chain reaction and in-situ hybridization has demonstrated the virus within several organs. Although diarrhea has been described in more than half of the cases of neonatal mortalities associated with SVA, there are no specific histological lesions in the intestine of affected animals. The virus is predominantly observed in association with the lamina propria vasculature, suggesting a viremic event rather than a primary injury to the intestinal epithelium (See Figure 1).
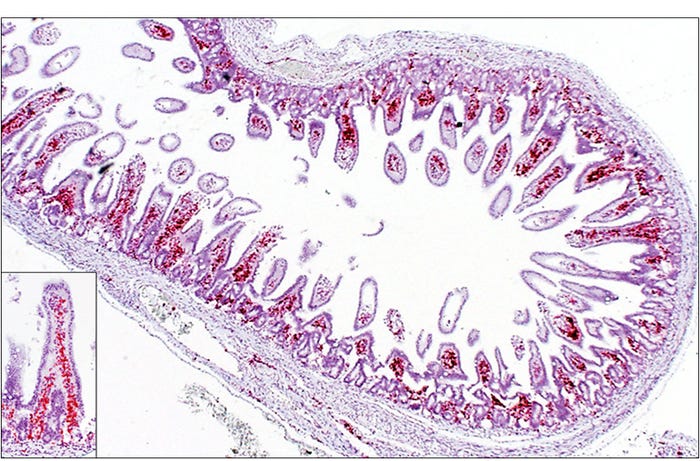
Figure 1: Piglet. Small intestine, Senecavirus A in-situ hybridization. Strong signals (red) representing SVA mRNA closely associated with the lamina propria vasculature. Inset — higher magnification
The general timeline of events for an individual infected pig is that it will develop viremia, or have virus detectable in the blood, within one to three days but this phase is relatively short and usually lasts for approximately one-and-a-half to two weeks. As the virus is disappearing from the blood, there is an increase in virus-specific antibodies, with detectable levels appearing around five to seven days and some reports of naturally infected herds have documented detectable levels out to 180 days after developing vesicles. Vesicles develop between two and seven days after infection and these skin lesions will usually heal within one to two weeks.
These vesicles and vesicular fluid have the highest concentration of virus but the virus is also shed in most secretions, with reports of PCR positive oral fluids, feces and semen. In experimental studies shedding is reported to start as early as one day after infection with detectable virus in the oral fluids and feces of some pigs at four weeks after infection. In one report, experimentally inoculated pigs which were no longer shedding virus had viral RNA detectable in the tonsil by PCR and ISH at 38 days post-inoculation1. The virus was also detected in the tonsils of naturally infected boars by PCR and ISH 91 days after clinical signs (see Figure 2). These findings have suggested a potential for SVA to establish a persistent infection or inducing an asymptomatic carrier state in infected animals.
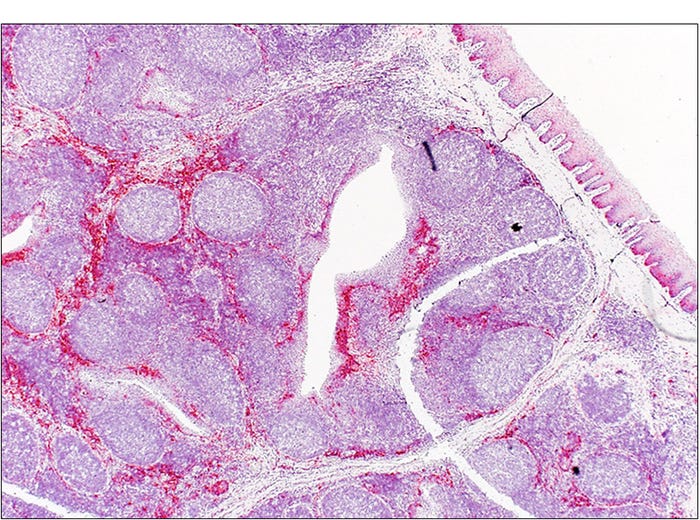
Figure 2: Boar. Tonsil. Senecavirus A in-situ hybridization. Strong signals (red) representing SVA mRNA in the tonsil of a naturally infected boar 91 days after clinical signs.
Immunity to subsequent SVA challenge after natural or experimental infection has not been reported to date. Sample collection from pigs with vesicular disease should only be performed by USDA-trained foreign animal disease diagnosticians. If you are monitoring a site that has already been confirmed to be infected with SVA these data suggest that PCR testing performed on serum (within the first week only), oral fluids and feces (within the first two to four weeks), or tonsil tissue (within the first five weeks, and perhaps even longer) of infected pigs should be positive and infected or previously infected pigs should be positive by serology (IFA or ELISA) starting around one week post-infection.
The definitive routes of transmission in natural infections have not been fully characterized but experimental infections have been successful using intranasal or combined intranasal/oral inoculation. It is likely that pigs are infected primarily through direct contact with infected pigs or exposure to secretions from infected pigs or contaminated fomites. Investigations of environmental samples, mice and flies obtained from infected farms showed that virus could be isolated from environmental samples (air, pens and crates), mouse feces and mouse intestines, and viral RNA was detected by PCR from flies2, which highlights the importance of proper disinfection of facilities previously housing infected pigs and raises the possibility of spread by non-swine species.
Disinfection of contaminated facilities or transport vehicles is an important step in preventing spread of the virus. The virus is a non-enveloped virus and very stable in the environment. A study of efficacy of three commonly used disinfectants showed that bleach (1:20 dilution) resulted in >99% neutralization on numerous substrates with contact times between five and 15 minutes. A quaternary ammonia compound plus aldehyde solution (Synergize; 1:256 dilution) and a phenolic disinfectant (Tek-Trol; 1:250 dilution) were less effective than bleach with 93% to 99% inactivation and 17% to 82% inactivation, respectively, after 60 minutes of contact time3. Hydrogen peroxide-based products (Virkon at 1% and Prevail at dilution 1:20) were also shown to be highly effective at 10 minutes contact time4.
While SVA continues to plague U.S. and global pork producers, it is important to be reminded of and understand some basic characteristics and behavior of this virus. SVA causes vesicular lesions affecting the skin, mouth and feet of pigs of all ages and has been associated with increased neonatal mortality which may be accompanied by neonatal diarrhea. If vesicular disease is present, your state animal health official must be notified in order to rule out other foreign animal diseases, such as FMD. The virus can be detected in multiple sample types but there is variability in the amount of time for which each sample type can be used for detection. Finally, SVA is extremely stable and contaminated facilities, transport vehicles and fomites are concerns for possible virus transmission but several disinfectants have been shown to be effective at neutralizing the virus.
Sources
1. Joshi LR, Fernandes MHV, Clement T, et al. Pathogenesis of Senecavirus A infection in finishing pigs. J Gen Virol. 2016; (2016):3267-3279. doi:10.1099/jgv.0.000631.
2. Joshi LR, Mohr KA, Clement T, et al. Detection of the emerging picornavirus senecavirus a in pigs, mice, and houseflies. J Clin Microbiol. 2016;54(6):1536-1545. doi:10.1128/JCM.03390-15.
3. Singh A, Mor S, Aboubakr H, Vannucci F, Patnayak D, Goyal S. Efficacy of three disinfectants against Senecacirus A on five surfaces and at two temperatures. J Swine Heal Prod. 2017;25(2):64-68.
4. Hole K, Ahmadpour F, Krishnan J, et al. Efficacy of accelerated hydrogen peroxide disinfectant on foot-and-mouth disease virus, swine vesicular disease virus and Senecavirus A. J Appl Microbiol 2017;122:634–639.
You May Also Like



