It is imperative that we develop effective antimicrobial alternatives that can support growth of pigs and poultry under various conditions.
April 11, 2017

By Michaela Trudeau, Chi Chen, Gerald Shurson, University of Minnesota Department of Animal Science; and Fernando Leite, Richard Isaacson and Pedro Urriola, University of Minnesota Department of Biomedical Veterinary Science
For more than 50 years, antibiotics were used in livestock feeding programs to effectively suppress pathogens and induce growth promotion of pigs and poultry (Jukes, 1948). The use of antibiotics at growth promoter levels offered a solution to subclinical diseases and other environmental stress (microbiome-host interactions) that decreased growth of food producing animals.
However, eliminating their usage in swine and poultry feeding programs has become necessary due to significant concerns of development of antimicrobial resistance (Andersson and Hughes, 2010). As result, newly enacted regulations in the United States prohibit the use of antibiotic growth promoters in diets for pigs and poultry. However, the increase in animal growth, control of subclinical diseases and other growth promotion benefits contributed to the overall efficiency of pork production and subsequently sustainability of food production. Therefore, it is imperative that we develop effective alternatives that can support growth of pigs and poultry under various conditions.
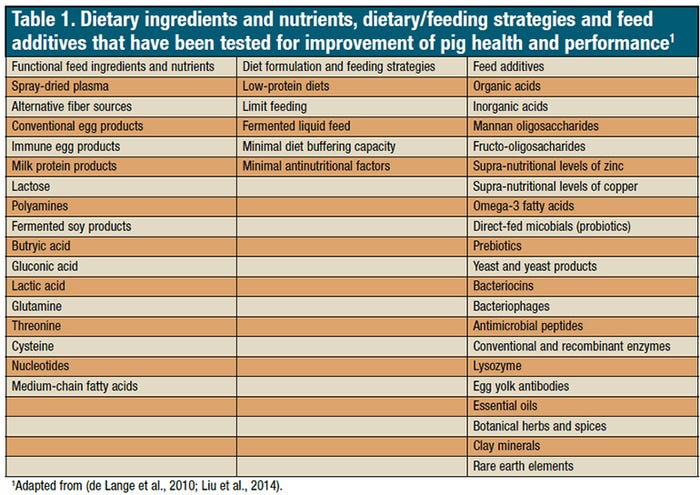
Currently, there are up to 20 categories of alternative products to growth promoters with claims of growth promotion and health improvement (Table 1). These products are added to pig diets without specific knowledge on their effectiveness and potential interactions with other dietary components. Such empirical use of alternative feed ingredients may decrease animal performance or increase unnecessary feeding cost.
A more mechanistic approach to evaluate and understand interactions between host and pathogens would allow producers to reach greater goals of production, animal welfare and disease control. At the center of the host pathogen interaction, there is a wide variety of poorly described metabolites. Likewise, the subsequent interaction of metabolites and host are poorly described (Figure 1).
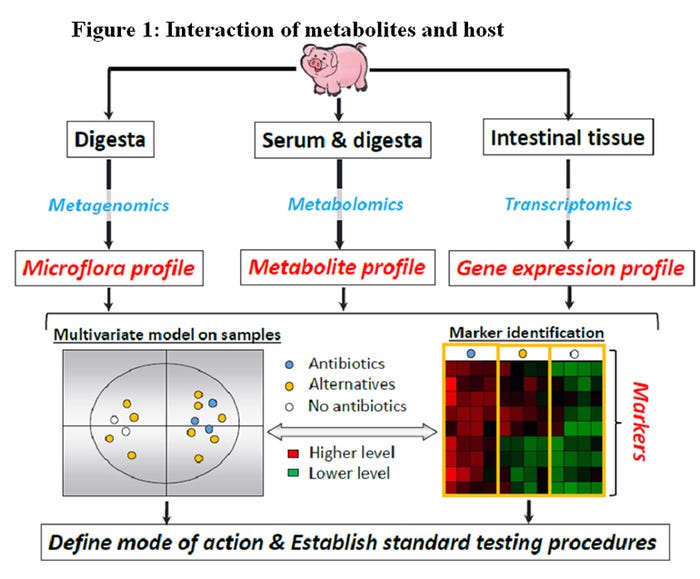
For evaluating and developing alternatives to antibiotics for growth promotion it is first necessary to understand their mechanism of action. What we currently know is that antibiotics impact the pig’s gut microbiota, intestinal health, nutrient utilization and ability to cope with disease pressures. What is still unclear is how these factors overlap with each other and the mechanism(s) involved.
Our ongoing research is starting to piece together changes that occur in the microbiome with those of metabolome in the gut i.e. what are the changes that occur in the bacterial population and how these changes relate with differences in the metabolites that are generated in the pig following growth promoter treatment.
The first experiment was conducted at two commercial farms to characterize the impact of dietary inclusion of tylosin (40 ppm), a common antibiotic growth promoter, on the population of bacteria in the gut. Treatment and monitoring of the microbiota began when pigs were 10 weeks of age and performed every three weeks until 22 weeks of age. A total of 12 differentially abundant bacterial genera were found in the feces from pigs in both farms. This included an increase of Sporacetigenium and Acetanaerobacterium, which are known to produce short chain acids. The most commonly described function of short chain fatty is as an energy source for the pig and are also involved in the maintenance of intestinal function (Liu, 2015). There was also an increase in Lactobacillus which when fed as a probiotic to pigs has been demonstrated to lead to increased growth performance and overall health (Valeriano et al., 2016). With this study, it was also possible to observe an interesting pattern in how the microbiome changed over time.
At 10 weeks, when treatment began, the microbiota of both treated and non-treated groups were similar, as was the case at the end of the trial when animals reached 22 weeks of age. What changed, however, was that the group which received tylosin had a shift in the microbiome at a much earlier time point (16 weeks) that was already like the microbiome at 22 weeks. This may indicate that along with specific changes in certain bacteria, the microbiome as a whole matures more rapidly when animals are given tylosin (Kim et al., 2012).
Changes in the microbiome from pigs was also associated to changes in relative abundance of metabolites in the pig’s feces. These data suggest that there are metabolic differences between pigs given antibiotics compared to non-antibiotic fed pigs. There is a multitude of metabolites that are different between pigs fed tylosin and the control diets, specifically with differences in the abundance of short-chain fatty acids, secondary bile acids and other yet to be identified metabolites.
Currently, more data are needed to support this hypothesis, but relating changes in metabolites to the gut microbiome allows for a more complete understanding and investigation of the impact that antibiotics have in enhancing growth. Without completely understanding the mechanism of increased growth, antibiotic alternatives could be used inappropriately without much added benefit. Understanding the mechanism of action will also allow producers to make informed decisions on which additives can be used together to produce synergistic effects. This systems biology approach of accounting for the connections between nutrition, microbiota and animal health will help to understand the mechanism for growth-promotion from antibiotics and help to develop alternatives to maximize efficiency.
References:
Andersson, D. I., and D. Hughes. 2010. Antibiotic resistance and its cost: is it possible to reverse resistance? Nat. Rev. Microbiol. 8:260–71. doi: 10.1038/nrmicro2319
de Lange, C. F. M., J. Pluske, J. Gong, and C. M. Nyachoti. 2010. Strategic use of feed ingredients and feed additives to stimulate gut health and development in young pigs. Livest. Sci. 134:124–134. doi: 10.1016/j.livsci.2010.06.117
Jukes, T. H. 1948. Jukes: Antibiotics in the Feeding of Domestic Animals. Ann. N.Y. Acad. Sci. 1:362–379.
Kim HB, Borewicz K, White BA, Singer RS, Sreevatsan S, Tu ZJ, Isaacson RE. Microbial shifts in the swine distal gut in response to the treatment with antimicrobial growth promoter, tylosin. Proc Natl Acad Sci U S A. 2012 Sep 18;109(38):15485-90.
Liu Y. Fatty acids, inflammation and intestinal health in pigs. J Anim Sci Biotechnol. 2015 Sep 9;6(1):41. doi: 10.1186/s40104-015-0040-1.
Liu, Y., M. Song, T. M. Che, J. J. Lee, D. Bravo, C. W. Maddox, and J. E. Pettigrew. 2014. Dietary plant extracts modulate gene expression profiles in ileal mucosa of weaned pigs after an Escherichia coli infection 1. :2050–2062. doi: 10.2527/jas2013-6422
Valeriano VD, Balolong MP, Kang DK. Probiotic roles of Lactobacillus sp. in swine: insights from gut microbiota. J Appl Microbiol. 2017 Mar;122(3):554-567. doi: 10.1111/jam.13364.
You May Also Like



