Study determines the pattern of protein deposition during gestation in an attempt to develop precision feeding recommendations for pregnant gilts.
March 1, 2017

By Crystal Levesque, South Dakota State University Department of Animal Science; E.G. Miller, University of Guelph Department of Animal Biosciences; Nathalie Trottier, Michigan State University Department of Animal Science; and C.F.M. de Lang, University of Guelph Department of Animal Biosciences
Precision livestock feeding (i.e. meeting nutrient requirements without excess, adjusted for growth rate and physiological state) allows optimal animal performance, while minimizing nutrient excretion and environmental risk. In swine production, ensuring optimal nutrient supply for gilts is of particular interest because poor reproductive performance in the first parity is a strong indicator of the lifetime reproductive potential. Therefore, optimizing gilt nutrition for successful pregnancy has long-term consequences for total production efficiency and sustainability of swine production.
However, there is limited information pertaining to the dynamics of protein deposition (Pd; nitrogen retention × 6.25) in gestating sows, which is a key determinant of amino acid requirements (National Research Council, 2012). Protein deposition can be separated into that associated with pregnancy (i.e. fetus, mammary gland, uterus, placenta and fluids) and that associated with maternal growth. While the amino acid requirements relative to pregnancy-associated tissues are reasonably characterized, data regarding the requirements for maternal Pd are severely limited. In late-gestation, the primary demand for nutrients is growth of the pregnancy-associated tissues, thus it is likely that maternal Pd is compromised toward the end of gestation due to increased fetal demands. However, the recently revised NRC (2012) nutrient requirement model assumes constant maternal Pd throughout gestation.
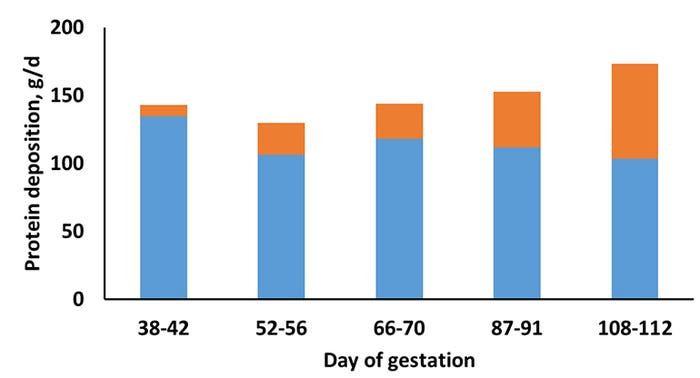
A study recently published in the Journal of Animal Science (Miller et al., 2016; JAS 94:3353-3361, doi.10.2527/jas.2016-0539) was conducted to determine whole-body and maternal Pd in gilts during gestation at two different feeding levels. Gilts were assigned to one of two feeding levels [15% below and above NRC (2012) energy intake recommendations] of a corn soybean-based diet (3.30 Mcal ME/kg; 0.82% SID Lys) at d 33 ± 0.5 of gestation. Nitrogen balance collections [N intake-N output in urine and feces=N balance (retained or lost)] were conducted at day 38, 52, 66, 87, and 108 ± 0.5 of gestation and the pattern of whole body N retention was characterized. Whole body retention was then separated into that associated with pregnancy, based on NRC (2012) equations which account for day of gestation, and that with maternal tissue.
Pregnancy-associated Pd was not affected by feeding level. Regardless of day of gestation, gilts at the higher feeding level had greater Pd (P < 0.001). There was no interaction between feeding level and day of gestation on whole body, pregnancy-associated, or maternal Pd; thus the patterns of Pd were consistent between the high and low feeding level. There was a positive linear and quadratic response (P < 0.001) for whole body and pregnancy-associated Pd; however, a negative linear and quadratic response (P < 0.001) for maternal Pd was observed (Figure 1, feeding levels grouped together).
In the current study, while the pattern of whole body Pd was both linear and quadratic, the quadratic response was a better fit for the data primarily due to a reduction in maternal Pd during mid-gestation, which may be related to a shift toward energy storage during this period to prepare for mobilization in late-gestation or lactation. In relation specifically to maternal Pd, the reduction in Pd in late-gestation may be related to a competition for nutrient supplies between maternal and fetal tissues that is regulated by the development of reduced insulin sensitivity in late-gestation as reported by Père et al. (2000).
Implications: The decline in maternal Pd in late-gestation, regardless of feeding level (i.e. not feed intake dependent), suggests some other physiological regulation of maternal Pd. The current NRC (2012) requirement model assumes constant maternal Pd throughout gestation. This study suggests that, at least for gilts, amino acid requirements and ideal amino acid ratio estimates in late-gestation may need to be revised to reflect a greater relative contribution of fetal tissue to the whole body amino acid requirement.
You May Also Like



