The objective of this study was to compare the efficacy of commercially available sources of MCFA and other fat sources versus a synthetic custom blend of MCFA to minimize the risk of PEDV cross-contamination as measured by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and bioassay.
December 17, 2016

Swine producers, veterinarians and researchers have been trying to find ways to stop the spread of porcine epidemic diarrhea ever since the virus was first identified in North American herds in May of 2013. Research has shown that PEDV has been able to survive completely in feed and individual feed ingredients.
Similar research has found that chemical treatments, such as medium chain fatty acids and commercial formaldehyde, have been effective in reducing the risk of PEDV cross-contaminating feed. However, the efficacy of individual MCFA levels is unknown.
The objective of this study was to compare the efficacy of commercially available sources of MCFA and other fat sources versus a synthetic custom blend of MCFA to minimize the risk of PEDV cross-contamination as measured by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and bioassay.
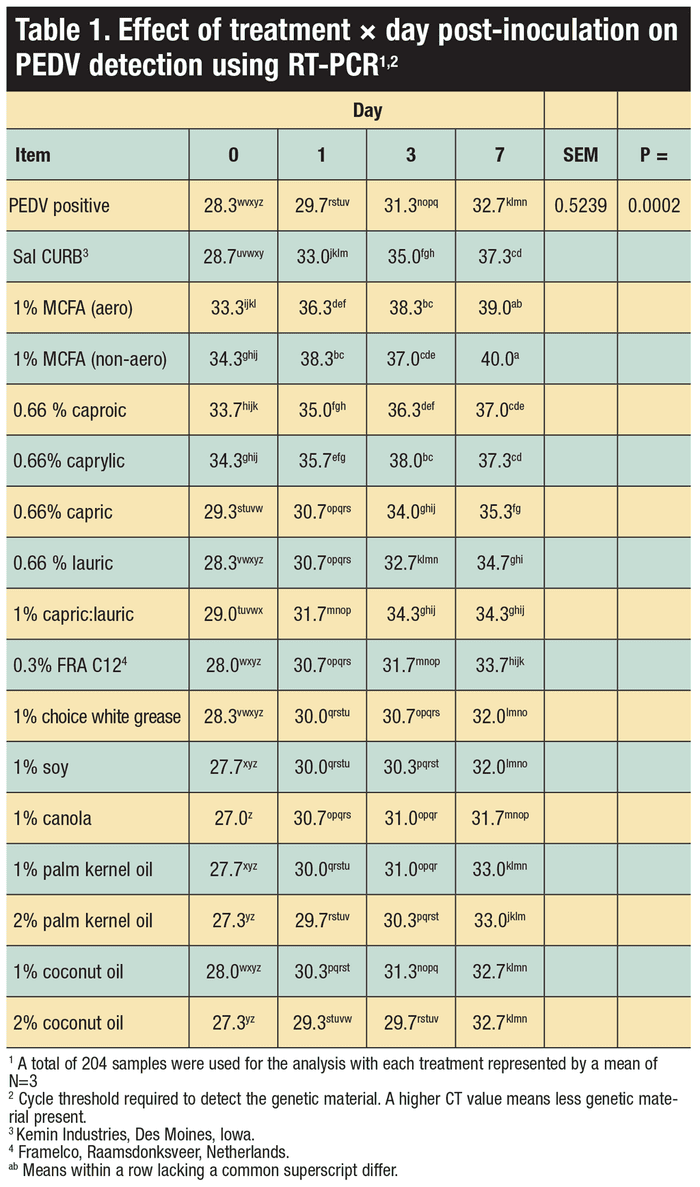
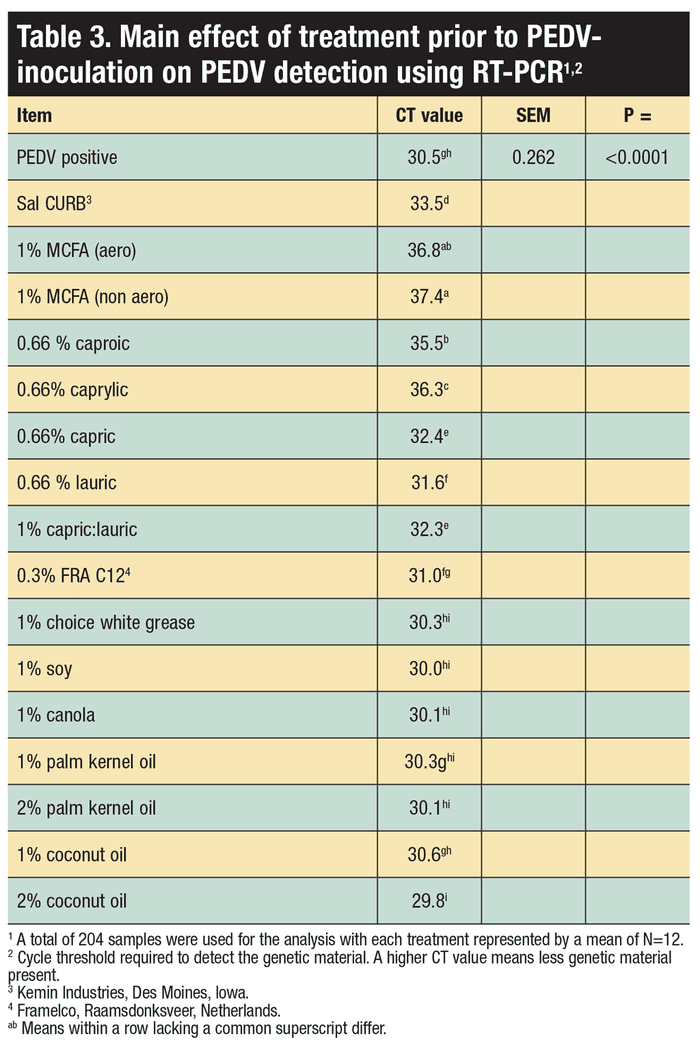
Seventeen treatments were applied to a swine diet, inoculated with PEDV and evaluated over four analysis days post inoculation. The 17 chemical treatments were: 1) Control inoculated with PEDV and no chemical treatment; 2) 0.3% commercial formaldehyde; 3) 1% MCFA [blend of caproic (C6), caprylic (C8) and capric acids (C10); 1:1:1] aerosolized into the feed or 4) placed directly into the feed; 5) 0.66% caproic acid; 6) 0.66% caprylic acid; 7) 0.66% capric acid; 8) 0.66% lauric acid (C12); 9) 1% capric and lauric acid mixture (1:1 ratio); 10) FRA C12 (Framelco, Raamsdonksveer, Netherlands); 11) 1% choice white grease; 12) 1% soy oil; 13) 1% canola oil; 14) 2% palm kernel oil; 15) 1% palm kernel oil; 16) 2% coconut oil; and 17) 1% coconut oil.
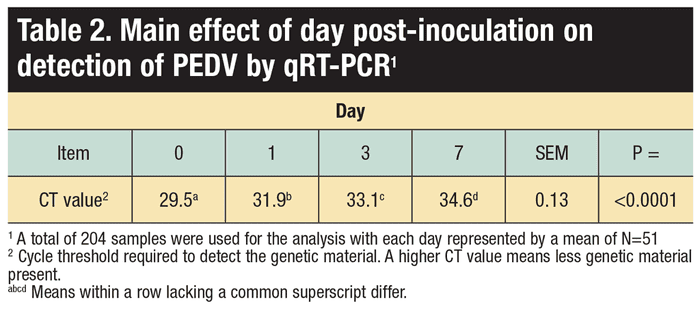
The four analysis days were zero-, one-, three- and seven-post inoculation.
Inoculation
The feed was inoculated using an appropriately sized pipet to allow even distribution of the virus within the feed. For the inoculation, 2.5 mL of diluted viral inoculum was placed in each 250 mL bottle containing 22.5 grams of each feed treatment, resulting in each bottle containing a PEDV concentration of 104 TCID50/g of feed. The bottles were then thoroughly shaken to ensure equal dispersion of the virus within each bottle. The samples were then stored at ambient temperature until aliquoted for viral RNA expression of PEDV at 0, one, three and seven days post inoculation via qRT-PCR. For each sample day, 100 mL of chilled PBS was placed in each 250 mL bottle containing 22.5 g of inoculated feed. Samples were then shaken to thoroughly mix and chilled at 4 degrees C overnight. Feed matrix supernatants, including two PCR samples and a bioassay sample, were then pulled and stored at -80 degrees C until the end of the trial.
Bioassay
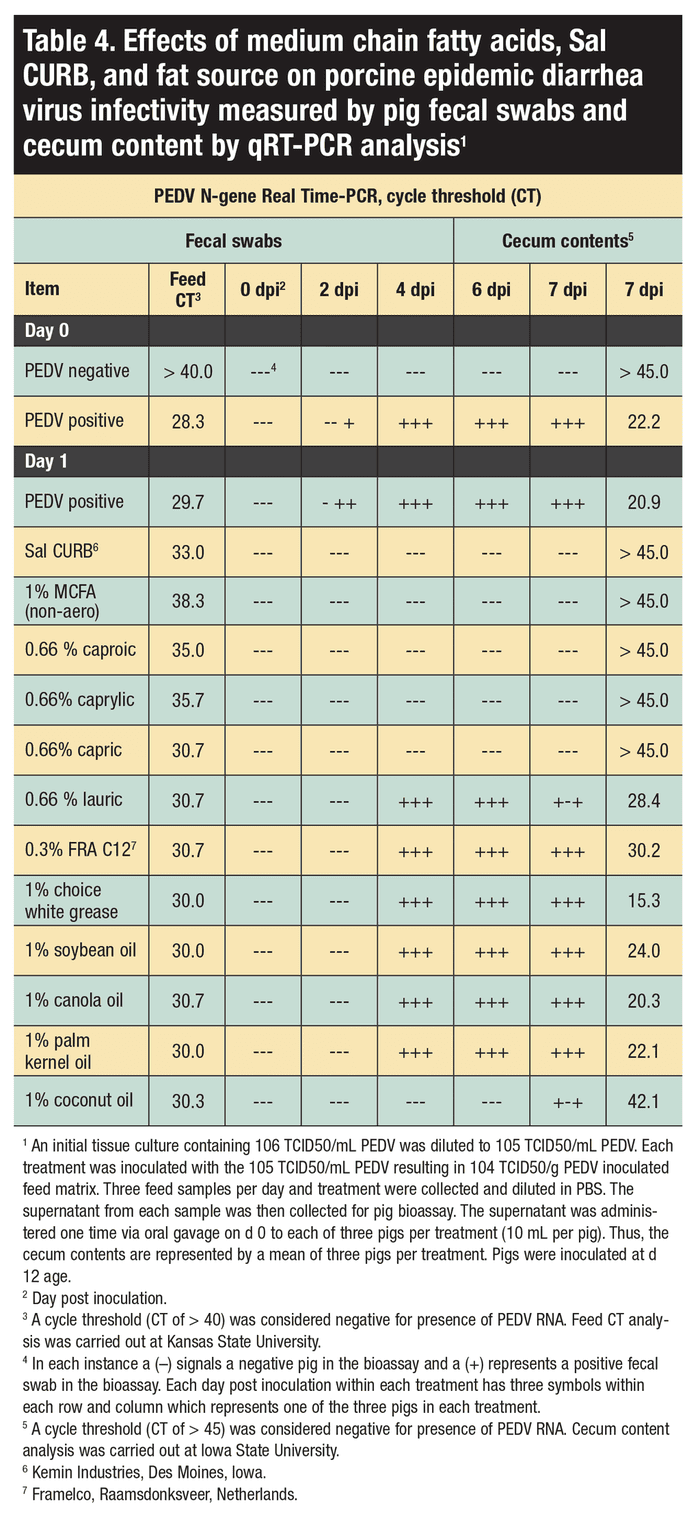
The Iowa State University Institutional Animal Care and Use Committee reviewed and approved the pig bioassay protocol. Based on the qRT-PCR results, 15 treatments were selected for the bioassay. The 15 treatments were 1) Day Zero negative control with no PEDV and no chemical treatment; 2) Day Zero positive control with PEDV and no chemical treatment; 3) Day 1 positive control with PEDV and no chemical treatment; 4) Day 1 0.3% Sal CURB; 5) Day 1 1% MCFA blend [caproic, caprylic and capric acids; 1:1:1] (non-aerosolized); 6) Day 1 1 0.66% caproic acid; 7) Day 1 0.66% caprylic acid; 8) Day 1 0.66% capric acid; 9) Day 1 0.66% lauric acid; 10) Day 1 FRA C12; 11) Day 1 1% choice white grease; 12) Day 1 1% soy oil; 13) Day 1 1% canola oil; 14) Day 1 1% palm kernel oil; and 15) Day 1 1% coconut oil.
A total of 45 crossbred, 10-day-old pigs of mixed sex were sourced from a single commercial, crossbred farrow-to-wean herd with no prior exposure to PEDV. Additionally, all pigs were confirmed negative for PEDV, porcine delta coronavirus and transmissible gastroenteritis virus based on fecal swab. To further confirm PEDV-negative status, collected blood serum was analyzed for PEDV antibodies by an indirect fluorescent antibody assay and TGEV antibodies by ELISA, both conducted at the Iowa State University Veterinary Diagnostic Laboratory. Pigs were allowed two days of adjustment to the new pens before the bioassay began. A total of 15 rooms (45 pigs) were assigned to treatment groups with one negative control room and 14 challenge rooms.
During bioassays, rectal swabs were collected on Days -2, zero, two, four, six and seven post-inoculation from all pigs and tested for PEDV RNA qRT-PCR. Following humane euthanasia at Day 7 post-inoculation, small intestine, cecum and colon samples were collected at necropsy, along with an aliquot of cecal contents. One section of formalin-fixed proximal, middle, distal jejunum and ileum was collected per pig for histopathology.
Results
Time, commercial formaldehyde, 1% MCFA mixture (1:1:1, caproic, caprylic and capric), 0.66% caproic (C6), 0.66% caprylic (C8) and 0.66% capric (C10) acids enhance the RNA degradation of PEDV in swine feed, and reduced PEDV infectivity. Notably, the MCFAs were as successful at mitigating PEDV as a commercially available formaldehyde-based product in the complete swine diet.
Study results suggest that a complete swine diet can be treated with MCFAs, specifically caproic (C6), caprylic (C8) and capric (C10) acids, as a means to reduce PEDV contamination in a complete swine diet and reduce PEDV infectivity. Although unproven, these compounds could be effective against other biologic hazards in feed as well. Also, it appears that some commercially available products that contain these MCFAs may be efficacious, but further investigation is needed to ensure dosage and composition are effective.
Researchers: R.A. Cochrane, S.S. Dritz, J.M. DeRouchey, M.D. Tokach, R.D. Goodband, J. Bia, Kansas State University Department of Diagnostic Medicine/Pathobiology, College of Veterinary Medicine; J.C. Woodworth, A.R. Huss, C.R. Stark, M. Saensukjaroenphon, Kansas State University Department of Grain Sciences and Industry, College of Agriculture; Q. Chen, J. Zhang, P.C. Gauger, R.J. Derscheid, R.G. Main, Iowa State University Veterinary Diagnostic and Production Animal Medicine, College of Veterinary Medicine; and C.K. Jones.
You May Also Like



