GlobalVetLink provides answers to the most frequently asked questions on veterinary feed directives.
March 2, 2017

The Food and Drug Administration’s new antibiotic rules mean many current over-the-counter “medically important” antibiotics, effective Jan. 1, now require a veterinary feed directive or veterinary prescription.
The fact remains veterinarians will now be writing more VFDs for antimicrobials in feed or prescriptions for antimicrobials in water. Speaking at the American Association of Swine Veterinarians annual meeting, Tyler Holck, DVM, GlobalVetLink Herd Health Management Solutions, illustrates the increasing number of VFDs in the GlobalVetLink system by species in Figure 1 and by the size of swine operation in Figure 2.
Figure 1
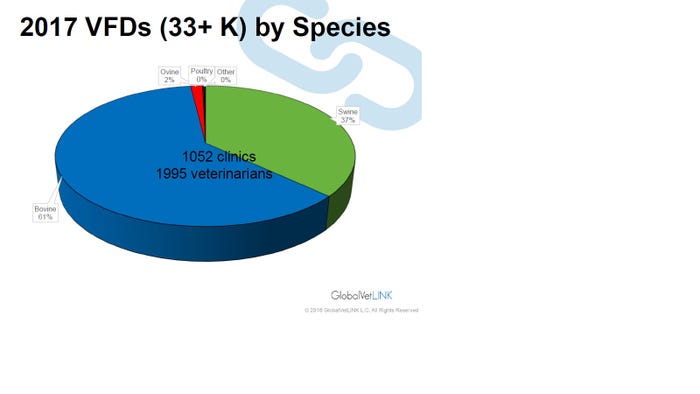
Figure 1
Figure 2
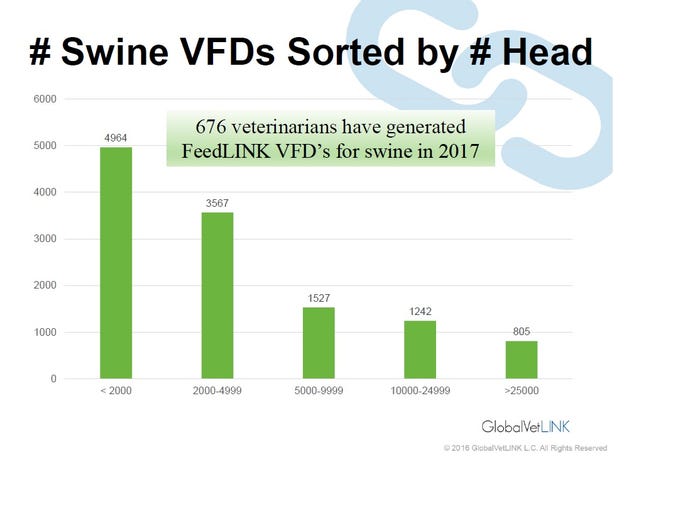
Figure 2
Veterinarians have a greater responsibility with the new FDA antibiotic rules. “So whether or not an animal health practitioner has been familiar with writing VFDs and/or scripts in the past, there is a need for understanding the changes and adjusting management strategies moving forward,” says Holck.
Still, changes are sure to cause confusion and questions surface as involved parties try to figure out the required documentation. At AASV, Holck shared answers he received from the FDA to the following frequently asked questions.
Q1: When converting milligrams per pound bodyweight doses to feed concentrations, are the concentrations required on the VFD?
A1: Yes, the VFD must include drug levels on a gram-per-ton basis.
Q2: How about the calculations?
A2: No, the VFD does not require the calculations the veterinarian may have performed to determine grams per ton. While not required, the information may be useful and can be included in the special instructions section.
Q3: Can a gram-per-ton range be listed on a VFD?
A3: Yes, the veterinarian may put the entire authorized range on the VFD.
Q4: Who is responsible for monitoring the appropriate amount of feed issued under a VFD?
A4: The distributor and client share responsibility for monitoring the amount of feed issued to fill a VFD. The client taking care of the animals should know their size and consumption rate and should share this information with the distributor to order an appropriate amount of feed. Quantities should be commensurate with the approximate number of animals.
Q5: For stage of production classifications that are general (i.e., swine) and there are no contraindications listed on the label, can a VFD be written for adult animals?
A5: Yes, if there is not language limiting the approval to a specific production class, the approval applies to all animals within the approved species, regardless of age.
Q6: U.S. market withdrawal times are required on a VFD, how should export market withdrawal times be handled on a VFD?
A6: The VFD is required to be filled out with the information that is on the U.S. drug approval. If it is necessary to include additional information specific to the care of the animals for export or other purposes, that information should be included on the VFD as special instructions.
Q7: For products/indications with an approved range of duration of use, can a VFD be written for a range?
A7: Yes, in situations where there is a range for the duration of use on the VFD drug approval, the veterinarian should use his or her medical judgment to determine an appropriate duration. It is acceptable for the veterinarian to indicate the approved range for the duration and then provide additional information on when it is appropriate to discontinue treatment.
You May Also Like



